Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis
- PMID: 23325411
- PMCID: PMC3625718
- DOI: 10.1152/ajpcell.00414.2012
Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis
Abstract
Chronic kidney disease, defined as loss of kidney function for more than three months, is characterized pathologically by glomerulosclerosis, interstitial fibrosis, tubular atrophy, peritubular capillary rarefaction, and inflammation. Recent studies have identified a previously poorly appreciated, yet extensive population of mesenchymal cells, called either pericytes when attached to peritubular capillaries or resident fibroblasts when embedded in matrix, as the progenitors of scar-forming cells known as myofibroblasts. In response to sustained kidney injury, pericytes detach from the vasculature and differentiate into myofibroblasts, a process not only causing fibrosis, but also directly contributing to capillary rarefaction and inflammation. The interrelationship of these three detrimental processes makes myofibroblasts and their pericyte progenitors an attractive target in chronic kidney disease. In this review, we describe current understanding of the mechanisms of pericyte-to-myofibroblast differentiation during chronic kidney disease, draw parallels with disease processes in the glomerulus, and highlight promising new therapeutic strategies that target pericytes or myofibroblasts. In addition, we describe the critical paracrine roles of epithelial, endothelial, and innate immune cells in the fibrogenic process.
Figures
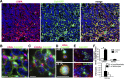
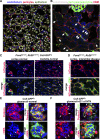

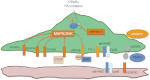
Similar articles
-
Novel insights into pericyte-myofibroblast transition and therapeutic targets in renal fibrosis.J Formos Med Assoc. 2012 Nov;111(11):589-98. doi: 10.1016/j.jfma.2012.09.008. Epub 2012 Oct 24. J Formos Med Assoc. 2012. PMID: 23217594 Review.
-
Kidney pericytes: a novel therapeutic target in interstitial fibrosis.Histol Histopathol. 2012 Dec;27(12):1503-14. doi: 10.14670/HH-27.1503. Histol Histopathol. 2012. PMID: 23059881 Review.
-
[Identification of Biomarkers for Tubular Injury and Interstitial Fibrosis in Chronic Kidney Disease].Yakugaku Zasshi. 2017;137(11):1355-1360. doi: 10.1248/yakushi.17-00150. Yakugaku Zasshi. 2017. PMID: 29093371 Review. Japanese.
-
Pivotal role of pericytes in kidney fibrosis.Clin Exp Pharmacol Physiol. 2011 Jul;38(7):467-73. doi: 10.1111/j.1440-1681.2011.05531.x. Clin Exp Pharmacol Physiol. 2011. PMID: 21517936 Free PMC article. Review.
-
Mechanisms of fibrosis: the role of the pericyte.Curr Opin Nephrol Hypertens. 2011 May;20(3):297-305. doi: 10.1097/MNH.0b013e328344c3d4. Curr Opin Nephrol Hypertens. 2011. PMID: 21422927 Review.
Cited by
-
Substrate-specific gene expression profiles in different kidney cell types are associated with Fabry disease.Mol Med Rep. 2015 Oct;12(4):5049-57. doi: 10.3892/mmr.2015.4010. Epub 2015 Jul 1. Mol Med Rep. 2015. PMID: 26135632 Free PMC article.
-
The Restoration of Vitamin D Levels Slows the Progression of Renal Ischemic Injury in Rats Previously Deficient in Vitamin D.Front Med (Lausanne). 2021 Apr 1;8:625647. doi: 10.3389/fmed.2021.625647. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33869246 Free PMC article.
-
Shear-wave elastography in renal stiffness in children with hematuria and/or proteinuria.Pediatr Res. 2024 Jul 3. doi: 10.1038/s41390-024-03363-5. Online ahead of print. Pediatr Res. 2024. PMID: 38961163
-
miR-122-5p Regulates Renal Fibrosis In Vivo.Int J Mol Sci. 2022 Dec 6;23(23):15423. doi: 10.3390/ijms232315423. Int J Mol Sci. 2022. PMID: 36499744 Free PMC article.
-
Macrophage polarization in tissue fibrosis.PeerJ. 2023 Oct 13;11:e16092. doi: 10.7717/peerj.16092. eCollection 2023. PeerJ. 2023. PMID: 37849830 Free PMC article. Review.
References
-
- Allison SJ. Podocyte biology: a new regulator of podocytes. Nat Rev Nephrol 8: 683, 2012 - PubMed
-
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs 169: 1–11, 2001 - PubMed
-
- Anders HJ. Toll-like receptors and danger signaling in kidney injury. J Am Soc Nephrol 21: 1270–1274, 2010 - PubMed
-
- Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21: 193–215, 2011 - PubMed
-
- Asada N, Takase M, Nakamura J, Oguchi A, Asada M, Suzuki N, Yamamura KI, Nagoshi N, Shibata S, Rao TN, Fehling HJ, Fukatsu A, Minegishi N, Kita T, Kimura T, Okano H, Yamamoto M, Yanagita M. Dysfunction of fibroblasts of extrarenal origin underlies renal fibrosis and renal anemia in mice. J Clin Invest 121: 3981–3990, 2011 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

