Role of connexins in metastatic breast cancer and melanoma brain colonization
- PMID: 23321642
- PMCID: PMC3625812
- DOI: 10.1242/jcs.112748
Role of connexins in metastatic breast cancer and melanoma brain colonization
Abstract
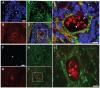
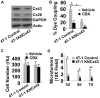
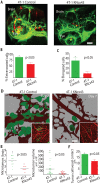
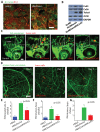
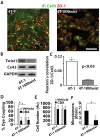
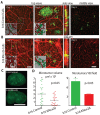
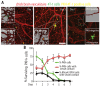
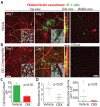
Breast cancer and melanoma cells commonly metastasize to the brain using homing mechanisms that are poorly understood. Cancer patients with brain metastases display poor prognosis and survival due to the lack of effective therapeutics and treatment strategies. Recent work using intravital microscopy and preclinical animal models indicates that metastatic cells colonize the brain, specifically in close contact with the existing brain vasculature. However, it is not known how contact with the vascular niche promotes microtumor formation. Here, we investigate the role of connexins in mediating early events in brain colonization using transparent zebrafish and chicken embryo models of brain metastasis. We provide evidence that breast cancer and melanoma cells utilize connexin gap junction proteins (Cx43, Cx26) to initiate brain metastatic lesion formation in association with the vasculature. RNAi depletion of connexins or pharmacological blocking of connexin-mediated cell-cell communication with carbenoxolone inhibited brain colonization by blocking tumor cell extravasation and blood vessel co-option. Activation of the metastatic gene twist in breast cancer cells increased Cx43 protein expression and gap junction communication, leading to increased extravasation, blood vessel co-option and brain colonization. Conversely, inhibiting twist activity reduced Cx43-mediated gap junction coupling and brain colonization. Database analyses of patient histories revealed increased expression of Cx26 and Cx43 in primary melanoma and breast cancer tumors, respectively, which correlated with increased cancer recurrence and metastasis. Together, our data indicate that Cx43 and Cx26 mediate cancer cell metastasis to the brain and suggest that connexins might be exploited therapeutically to benefit cancer patients with metastatic disease.
Figures
Similar articles
-
Correlations of differentially expressed gap junction connexins Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and prognosis.PLoS One. 2014 Nov 10;9(11):e112541. doi: 10.1371/journal.pone.0112541. eCollection 2014. PLoS One. 2014. PMID: 25383624 Free PMC article.
-
Dysregulation of lncRNA-CCRR contributes to brain metastasis of breast cancer by intercellular coupling via regulating connexin 43 expression.J Cell Mol Med. 2021 May;25(10):4826-4834. doi: 10.1111/jcmm.16455. Epub 2021 Apr 1. J Cell Mol Med. 2021. PMID: 33793070 Free PMC article.
-
Connexin43 reduces melanoma growth within a keratinocyte microenvironment and during tumorigenesis in vivo.J Biol Chem. 2014 Jan 17;289(3):1592-603. doi: 10.1074/jbc.M113.507228. Epub 2013 Dec 2. J Biol Chem. 2014. PMID: 24297173 Free PMC article.
-
Expression pattern of different gap junction connexins is related to embryo implantation.Int J Dev Biol. 1996 Feb;40(1):361-7. Int J Dev Biol. 1996. PMID: 8735949 Review.
-
Gap junction connexins in female reproductive organs: implications for women's reproductive health.Hum Reprod Update. 2015 May-Jun;21(3):340-52. doi: 10.1093/humupd/dmv007. Epub 2015 Feb 9. Hum Reprod Update. 2015. PMID: 25667189 Review.
Cited by
-
Connexin 43 expression predicts poor progression-free survival in patients with non-muscle invasive urothelial bladder cancer.J Clin Pathol. 2015 Oct;68(10):819-24. doi: 10.1136/jclinpath-2015-202898. Epub 2015 Aug 6. J Clin Pathol. 2015. PMID: 26251520 Free PMC article.
-
Metastasis prevention by targeting the dormant niche.Nat Rev Cancer. 2015 Apr;15(4):238-47. doi: 10.1038/nrc3910. Nat Rev Cancer. 2015. PMID: 25801619 Free PMC article. Review.
-
Prevalence and Clinicopathologic Features of Canine Metastatic Melanoma Involving the Central Nervous System: A Retrospective Analysis and Comparative Review.Front Oncol. 2022 May 27;12:868004. doi: 10.3389/fonc.2022.868004. eCollection 2022. Front Oncol. 2022. PMID: 35692802 Free PMC article.
-
Eukaryotic Translation Initiation Factor 5A (EIF5A) Regulates Pancreatic Cancer Metastasis by Modulating RhoA and Rho-associated Kinase (ROCK) Protein Expression Levels.J Biol Chem. 2015 Dec 11;290(50):29907-19. doi: 10.1074/jbc.M115.687418. Epub 2015 Oct 19. J Biol Chem. 2015. PMID: 26483550 Free PMC article.
-
GJA1 rs2071165 A > G Variant Increased Gastric Cancer Risk in Females of Northwest China: A Case-Control Study.J Oncol. 2021 May 19;2021:5556303. doi: 10.1155/2021/5556303. eCollection 2021. J Oncol. 2021. PMID: 34221012 Free PMC article.
References
-
- Barkan D., Kleinman H., Simmons J. L., Asmussen H., Kamaraju A. K., Hoenorhoff M. J., Liu Z. Y., Costes S. V., Cho E. H., Lockett S.et al. (2008). Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 68, 6241–6250 10.1158/0008-5472.CAN-07-6849 - DOI - PMC - PubMed
-
- Bauer J., Margolis M., Schreiner C., Edgell C. J., Azizkhan J., Lazarowski E., Juliano R. L. (1992). In vitro model of angiogenesis using a human endothelium-derived permanent cell line: contributions of induced gene expression, G-proteins, and integrins. J. Cell. Physiol. 153, 437–449 10.1002/jcp.1041530302 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

