TLR-4 engagement of dendritic cells confers a BST-2/tetherin-mediated restriction of HIV-1 infection to CD4+ T cells across the virological synapse
- PMID: 23311681
- PMCID: PMC3561259
- DOI: 10.1186/1742-4690-10-6
TLR-4 engagement of dendritic cells confers a BST-2/tetherin-mediated restriction of HIV-1 infection to CD4+ T cells across the virological synapse
Abstract
Background: Dendritic cells and their subsets, located at mucosal surfaces, are among the first immune cells to encounter disseminating pathogens. The cellular restriction factor BST-2/tetherin (also known as CD317 or HM1.24) potently restricts HIV-1 release by retaining viral particles at the cell surface in many cell types, including primary cells such as macrophages. However, BST-2/tetherin does not efficiently restrict HIV-1 infection in immature dendritic cells.
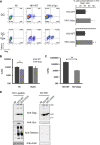
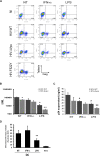
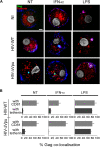
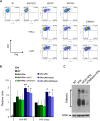
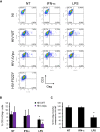
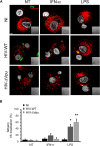
Results: We now report that BST-2/tetherin expression in myeloid (myDC) and monocyte-derived dendritic cells (DC) can be significantly up-regulated by IFN-α treatment and TLR-4 engagement with LPS. In contrast to HeLa or 293T cells, infectious HIV-1 release in immature DC and IFN-α-matured DC was only modestly affected in the absence of Vpu compared to wild-type viruses. Strikingly, immunofluorescence analysis revealed that BST-2/tetherin was excluded from HIV containing tetraspanin-enriched microdomains (TEMs) in both immature DC and IFN-α-matured DC. In contrast, in LPS-mediated mature DC, BST-2/tetherin exerted a significant restriction in transfer of HIV-1 infection to CD4+ T cells. Additionally, LPS, but not IFN-α stimulation of immature DC, leads to a dramatic redistribution of cellular restriction factors to the TEM as well as at the virological synapse between DC and CD4+ T cells.
Conclusions: In conclusion, we demonstrate that TLR-4 engagement in immature DC significantly up-regulates the intrinsic antiviral activity of BST-2/tetherin, during cis-infection of CD4+ T cells across the DC/T cell virological synapse. Manipulating the function and potency of cellular restriction factors such as BST-2/tetherin to HIV-1 infection, has implications in the design of antiviral therapeutic strategies.
Figures
Similar articles
-
Tetherin does not significantly restrict dendritic cell-mediated HIV-1 transmission and its expression is upregulated by newly synthesized HIV-1 Nef.Retrovirology. 2011 Apr 19;8:26. doi: 10.1186/1742-4690-8-26. Retrovirology. 2011. PMID: 21504576 Free PMC article.
-
Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells.J Virol. 2010 Dec;84(23):12185-99. doi: 10.1128/JVI.01447-10. Epub 2010 Sep 22. J Virol. 2010. PMID: 20861257 Free PMC article.
-
Emerging role of the host restriction factor tetherin in viral immune sensing.J Mol Biol. 2013 Dec 13;425(24):4956-64. doi: 10.1016/j.jmb.2013.09.029. Epub 2013 Sep 26. J Mol Biol. 2013. PMID: 24075872 Review.
-
Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism.J Virol. 2009 Aug;83(16):7931-47. doi: 10.1128/JVI.00242-09. Epub 2009 Jun 10. J Virol. 2009. PMID: 19515779 Free PMC article.
-
Antiviral activity of the interferon-induced cellular protein BST-2/tetherin.AIDS Res Hum Retroviruses. 2009 Dec;25(12):1197-210. doi: 10.1089/aid.2009.0253. AIDS Res Hum Retroviruses. 2009. PMID: 19929170 Free PMC article. Review.
Cited by
-
PolyICLC Exerts Pro- and Anti-HIV Effects on the DC-T Cell Milieu In Vitro and In Vivo.PLoS One. 2016 Sep 7;11(9):e0161730. doi: 10.1371/journal.pone.0161730. eCollection 2016. PLoS One. 2016. PMID: 27603520 Free PMC article.
-
Distinct Requirements for HIV-1 Accessory Proteins during Cell Coculture and Cell-Free Infection.Viruses. 2019 Apr 26;11(5):390. doi: 10.3390/v11050390. Viruses. 2019. PMID: 31027334 Free PMC article.
-
Identification of Host Trafficking Genes Required for HIV-1 Virological Synapse Formation in Dendritic Cells.J Virol. 2020 Apr 16;94(9):e01597-19. doi: 10.1128/JVI.01597-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32075937 Free PMC article.
-
Tetherin can restrict cell-free and cell-cell transmission of HIV from primary macrophages to T cells.PLoS Pathog. 2014 Jul 3;10(7):e1004189. doi: 10.1371/journal.ppat.1004189. eCollection 2014 Jul. PLoS Pathog. 2014. PMID: 24991932 Free PMC article.
-
CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies.PLoS Pathog. 2015 Mar 11;11(3):e1004751. doi: 10.1371/journal.ppat.1004751. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25760631 Free PMC article.
References
-
- Blanchet FP, Moris A, Nikolic DS, Lehmann M, Cardinaud S, Stalder R, Garcia E, Dinkins C, Leuba F, Wu L, Schwartz O, Deretic V, Piguet V. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity. 2010;32(5):654–669. doi: 10.1016/j.immuni.2010.04.011. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

