CRF01_AE-specific neutralizing activity observed in plasma derived from HIV-1-infected Thai patients residing in northern Thailand: comparison of neutralizing breadth and potency between plasma derived from rapid and slow progressors
- PMID: 23308290
- PMCID: PMC3538751
- DOI: 10.1371/journal.pone.0053920
CRF01_AE-specific neutralizing activity observed in plasma derived from HIV-1-infected Thai patients residing in northern Thailand: comparison of neutralizing breadth and potency between plasma derived from rapid and slow progressors
Abstract
Background: Development of a protective vaccine against human immunodeficiency virus type 1 (HIV-1) is an important subject in the field of medical sciences; however, it has not yet been achieved. Potent and broadly neutralizing antibodies are found in the plasma of some HIV-1-infected patients, whereas such antibody responses have failed to be induced by currently used vaccine antigens. In order to develop effective vaccine antigens, it is important to reveal the molecular mechanism of how strong humoral immune responses are induced in infected patients. As part of such studies, we examined the correlation between the anti-HIV-1 neutralizing antibody response and disease progression.
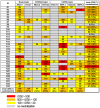
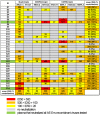
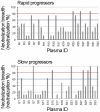
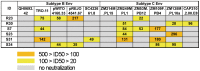
Methodology/principal findings: We evaluated the anti-HIV-1 neutralizing activity of plasma derived from 33 rapid and 34 slow progressors residing in northern Thailand. The level of neutralizing activity varied considerably among plasmas, and no statistically significant differences in the potency and breadth of neutralizing activities were observed overall between plasma derived from rapid and slow progressors; however, plasma of 4 slow progressors showed neutralizing activity against all target viruses, whereas none of the plasma of rapid progressors showed such neutralizing activity. In addition, 21% and 9% of plasmas derived from slow and rapid progressors inhibited the replication of more than 80% of CRF01_AE Env-recombinant viruses tested, respectively. Neutralization of subtype B and C Env-recombinant viruses by the selected plasma was also examined; however, these plasma samples inhibited the replication of only a few viruses tested.
Conclusions/significance: Although no statistically significant differences were observed in the potency and breadth of anti-HIV-1 neutralizing activities between plasma derived from rapid and slow progressors, several plasma samples derived from slow progressors neutralized CRF01_AE Env-recombinant viruses more frequently than those from rapid progressors. In addition, plasma derived from HIV-1-infected Thai patients showed CRF01_AE-specific neutralizing activity.
Conflict of interest statement
Figures
Similar articles
-
Neutralization breadth and potency of serum derived from recently human immunodeficiency virus type 1-infected Thai individuals.Microbes Infect. 2016 May;18(5):346-53. doi: 10.1016/j.micinf.2015.12.009. Epub 2016 Jan 14. Microbes Infect. 2016. PMID: 26774333
-
Profiles of neutralizing antibody response in chronically human immunodeficiency virus type 1 clade B'-infected former plasma donors from China naive to antiretroviral therapy.J Gen Virol. 2012 Oct;93(Pt 10):2267-2278. doi: 10.1099/vir.0.043802-0. Epub 2012 Jul 12. J Gen Virol. 2012. PMID: 22791603
-
Phenotypic studies on recombinant human immunodeficiency virus type 1 (HIV-1) containing CRF01_AE env gene derived from HIV-1-infected patient, residing in central Thailand.Microbes Infect. 2009 Mar;11(3):334-43. doi: 10.1016/j.micinf.2008.12.008. Epub 2008 Dec 27. Microbes Infect. 2009. PMID: 19136072
-
Specificity of the autologous neutralizing antibody response.Curr Opin HIV AIDS. 2009 Sep;4(5):358-63. doi: 10.1097/COH.0b013e32832ea7e8. Curr Opin HIV AIDS. 2009. PMID: 20048698 Free PMC article. Review.
-
Engineering multi-specific antibodies against HIV-1.Retrovirology. 2018 Aug 29;15(1):60. doi: 10.1186/s12977-018-0439-9. Retrovirology. 2018. PMID: 30157871 Free PMC article. Review.
Cited by
-
Impact of amino acid substitutions in the V2 and C2 regions of human immunodeficiency virus type 1 CRF01_AE envelope glycoprotein gp120 on viral neutralization susceptibility to broadly neutralizing antibodies specific for the CD4 binding site.Retrovirology. 2014 Apr 23;11:32. doi: 10.1186/1742-4690-11-32. Retrovirology. 2014. PMID: 24758333 Free PMC article.
-
CRF19_cpx is an Evolutionary fit HIV-1 Variant Strongly Associated With Rapid Progression to AIDS in Cuba.EBioMedicine. 2015 Jan 28;2(3):244-54. doi: 10.1016/j.ebiom.2015.01.015. eCollection 2015 Mar. EBioMedicine. 2015. PMID: 26137563 Free PMC article.
-
HIV-1 autologous antibody neutralization associates with mother to child transmission.PLoS One. 2013 Jul 17;8(7):e69274. doi: 10.1371/journal.pone.0069274. Print 2013. PLoS One. 2013. PMID: 23874931 Free PMC article.
-
Humoral Antibody Responses to HIV Viral Proteins and to CD4 Among HIV Controllers, Rapid and Typical Progressors in an HIV-Positive Patient Cohort.AIDS Res Hum Retroviruses. 2016 Dec;32(12):1187-1197. doi: 10.1089/AID.2016.0182. Epub 2016 Nov 21. AIDS Res Hum Retroviruses. 2016. PMID: 27771962 Free PMC article.
References
-
- Girard MP, Osmanov S, Assossou OM, Kieny MP (2011) Human immunodeficiency virus (HIV) immunopathogenesis and vaccine development: A review. Vaccine 29: 6191–6218. - PubMed
-
- Barbas CF 3rd, Collet TA, Amberg W, Roben P, Binley JM, et al (1993) Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol 230: 812–823. - PubMed
-
- Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, et al. (2001) A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS research and human retroviruses 17: 1757–1765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

