Daxx upregulation within the cytoplasm of reovirus-infected cells is mediated by interferon and contributes to apoptosis
- PMID: 23302889
- PMCID: PMC3592169
- DOI: 10.1128/JVI.02324-12
Daxx upregulation within the cytoplasm of reovirus-infected cells is mediated by interferon and contributes to apoptosis
Abstract
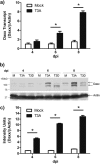
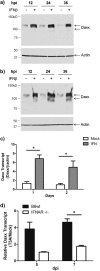
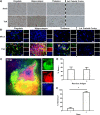
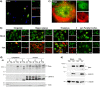
Reovirus infection is a well-characterized experimental system for the study of viral pathogenesis and antiviral immunity within the central nervous system (CNS). We have previously shown that c-Jun N-terminal kinase (JNK) and the Fas death receptor each play a role in neuronal apoptosis occurring in reovirus-infected brains. Death-associated protein 6 (Daxx) is a cellular protein that mechanistically links Fas signaling to JNK signaling in several models of apoptosis. In the present study, we demonstrate that Daxx is upregulated in reovirus-infected brain tissue through a type I interferon-mediated mechanism. Daxx upregulation is limited to brain regions that undergo reovirus-induced apoptosis and occurs in the cytoplasm and nucleus of neurons. Cytoplasmic Daxx is present in Fas-expressing cells during reovirus encephalitis, suggesting a role for Daxx in Fas-mediated apoptosis following reovirus infection. Further, in vitro expression of a dominant negative form of Daxx (DN-Daxx), which binds to Fas but which does not transmit downstream signaling, inhibits apoptosis of reovirus-infected cells. In contrast, in vitro depletion of Daxx results in increased expression of caspase 3 and apoptosis, suggesting that Daxx plays an antiapoptotic role in the nucleus. Overall, these data imply a regulatory role for Daxx in reovirus-induced apoptosis, depending on its location in the nucleus or cytoplasm.
Figures




Similar articles
-
Fas-mediated apoptotic signaling in the mouse brain following reovirus infection.J Virol. 2009 Jun;83(12):6161-70. doi: 10.1128/JVI.02488-08. Epub 2009 Mar 25. J Virol. 2009. PMID: 19321603 Free PMC article.
-
Apoptosis signal-regulating kinase 1 controls the proapoptotic function of death-associated protein (Daxx) in the cytoplasm.J Biol Chem. 2001 Oct 19;276(42):39103-6. doi: 10.1074/jbc.M105928200. Epub 2001 Aug 8. J Biol Chem. 2001. PMID: 11495919
-
4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to Fas.Biochemistry. 2008 Jan 8;47(1):143-56. doi: 10.1021/bi701559f. Epub 2007 Dec 11. Biochemistry. 2008. PMID: 18069800 Free PMC article.
-
Daxx: death or survival protein?Trends Cell Biol. 2006 Feb;16(2):97-104. doi: 10.1016/j.tcb.2005.12.002. Epub 2006 Jan 10. Trends Cell Biol. 2006. PMID: 16406523 Review.
-
PML NBs (ND10) and Daxx: from nuclear structure to protein function.Front Biosci. 2008 May 1;13:7132-42. doi: 10.2741/3216. Front Biosci. 2008. PMID: 18508722 Review.
Cited by
-
DAXX mediates high phosphate-induced endothelial cell apoptosis in vitro through activating ERK signaling.PeerJ. 2020 Jun 19;8:e9203. doi: 10.7717/peerj.9203. eCollection 2020. PeerJ. 2020. PMID: 32596036 Free PMC article.
-
Mitochondrial p53 Contributes to Reovirus-Induced Neuronal Apoptosis and Central Nervous System Injury in a Mouse Model of Viral Encephalitis.J Virol. 2016 Aug 12;90(17):7684-91. doi: 10.1128/JVI.00583-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27307572 Free PMC article.
-
Death Domain-Associated Protein Promotes Colon Cancer Metastasis through Direct Interaction with ZEB1.J Cancer. 2020 Jan 1;11(3):750-758. doi: 10.7150/jca.34233. eCollection 2020. J Cancer. 2020. PMID: 31942198 Free PMC article.
-
Virion factors that target Daxx to overcome intrinsic immunity.J Virol. 2013 Oct;87(19):10412-22. doi: 10.1128/JVI.00425-13. Epub 2013 Jul 17. J Virol. 2013. PMID: 23864634 Free PMC article. Review.
-
Gut Transcription in Helicoverpa zea is Dynamically Altered in Response to Baculovirus Infection.Insects. 2013 Sep 23;4(3):506-20. doi: 10.3390/insects4030506. Insects. 2013. PMID: 26462433 Free PMC article.
References
-
- Tan K, Patel S, Gandhi N, Chow F, Rumbaugh J, Nath A. 2008. Burden of neuroinfectious diseases on the neurology service in a tertiary care center. Neurology 71:1160–1166 - PubMed
-
- Tyler K. L. 2004. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret's. Herpes 11:57A–64A - PubMed
-
- Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, Lebon P, Canton P, Rozenberg F. 2002. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin. Infect. Dis. 35:254–260 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

