Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo
- PMID: 23300790
- PMCID: PMC3530470
- DOI: 10.1371/journal.pone.0052830
Reconstitution of mitochondria derived vesicle formation demonstrates selective enrichment of oxidized cargo
Abstract
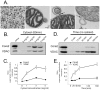
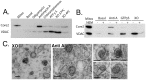
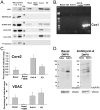
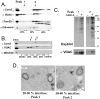
The mechanisms that ensure the removal of damaged mitochondrial proteins and lipids are critical for the health of the cell, and errors in these pathways are implicated in numerous degenerative diseases. We recently uncovered a new pathway for the selective removal of proteins mediated by mitochondrial derived vesicular carriers (MDVs) that transit to the lysosome. However, it was not determined whether these vesicles were selectively enriched for oxidized, or damaged proteins, and the extent to which the complexes of the electron transport chain and the mtDNA-containing nucloids may have been incorporated. In this study, we have developed a cell-free mitochondrial budding reaction in vitro in order to better dissect the pathway. Our data confirm that MDVs are stimulated upon various forms of mitochondrial stress, and the vesicles incorporated quantitative amounts of cargo, whose identity depended upon the nature of the stress. Under the conditions examined, MDVs did not incorporate complexes I and V, nor were any nucleoids present, demonstrating the specificity of cargo incorporation. Stress-induced MDVs are selectively enriched for oxidized proteins, suggesting that conformational changes induced by oxidation may initiate their incorporation into the vesicles. Ultrastructural analyses of MDVs isolated on sucrose flotation gradients revealed the formation of both single and double membranes vesicles of unique densities and uniform diameter. This work provides a framework for a reductionist approach towards a detailed examination of the mechanisms of MDV formation and cargo incorporation, and supports the emerging concept that MDVs are critical contributors to mitochondrial quality control.
Conflict of interest statement
Figures
Similar articles
-
Proteomics characterization of mitochondrial-derived vesicles under oxidative stress.FASEB J. 2021 Apr;35(4):e21278. doi: 10.1096/fj.202002151R. FASEB J. 2021. PMID: 33769614 Free PMC article.
-
A vesicular transport pathway shuttles cargo from mitochondria to lysosomes.Curr Biol. 2012 Jan 24;22(2):135-41. doi: 10.1016/j.cub.2011.11.057. Epub 2012 Jan 5. Curr Biol. 2012. PMID: 22226745
-
Proteomic Profiling of Mitochondrial-Derived Vesicles in Brain Reveals Enrichment of Respiratory Complex Sub-assemblies and Small TIM Chaperones.J Proteome Res. 2021 Jan 1;20(1):506-517. doi: 10.1021/acs.jproteome.0c00506. Epub 2020 Nov 26. J Proteome Res. 2021. PMID: 33242952
-
Mitochondrial-derived vesicles: Gatekeepers of mitochondrial response to oxidative stress.Free Radic Biol Med. 2022 Aug 1;188:185-193. doi: 10.1016/j.freeradbiomed.2022.06.233. Epub 2022 Jun 21. Free Radic Biol Med. 2022. PMID: 35750270 Review.
-
Mitochondrial-derived vesicles: Recent insights.J Cell Mol Med. 2022 Jun;26(12):3323-3328. doi: 10.1111/jcmm.17391. Epub 2022 May 18. J Cell Mol Med. 2022. PMID: 35582908 Free PMC article. Review.
Cited by
-
New insights into the interplay between autophagy and oxidative and endoplasmic reticulum stress in neuronal cell death and survival.Front Cell Dev Biol. 2022 Sep 16;10:994037. doi: 10.3389/fcell.2022.994037. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36187470 Free PMC article. Review.
-
Teaching the basics of autophagy and mitophagy to redox biologists--mechanisms and experimental approaches.Redox Biol. 2015;4:242-59. doi: 10.1016/j.redox.2015.01.003. Epub 2015 Jan 13. Redox Biol. 2015. PMID: 25618581 Free PMC article. Review.
-
Mitochondrial-derived vesicles retain membrane potential and contain a functional ATP synthase.EMBO Rep. 2023 May 4;24(5):e56114. doi: 10.15252/embr.202256114. Epub 2023 Mar 17. EMBO Rep. 2023. PMID: 36929726 Free PMC article.
-
Voltage-Dependent Anion Selective Channel 3: Unraveling Structural and Functional Features of the Least Known Porin Isoform.Front Physiol. 2022 Jan 10;12:784867. doi: 10.3389/fphys.2021.784867. eCollection 2021. Front Physiol. 2022. PMID: 35082690 Free PMC article. Review.
-
Generation and Release of Mitochondrial-Derived Vesicles in Health, Aging and Disease.J Clin Med. 2020 May 12;9(5):1440. doi: 10.3390/jcm9051440. J Clin Med. 2020. PMID: 32408624 Free PMC article. Review.
References
-
- Arnold I, Langer T (2002) Membrane protein degradation by AAA proteases in mitochondria. Biochim Biophys Acta 1592: 89–96. - PubMed
-
- Augustin S, Nolden M, Muller S, Hardt O, Arnold I, et al. (2005) Characterization of peptides released from mitochondria: evidence for constant proteolysis and peptide efflux. J Biol Chem 280: 2691–2699. - PubMed
-
- Bota DA, Davies KJ (2002) Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol 4: 674–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

