Cardiomyocyte-specific overexpression of HEXIM1 prevents right ventricular hypertrophy in hypoxia-induced pulmonary hypertension in mice
- PMID: 23300697
- PMCID: PMC3534105
- DOI: 10.1371/journal.pone.0052522
Cardiomyocyte-specific overexpression of HEXIM1 prevents right ventricular hypertrophy in hypoxia-induced pulmonary hypertension in mice
Abstract
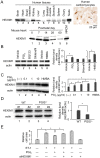
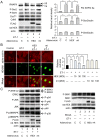
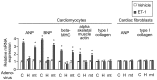
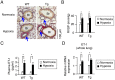
Right ventricular hypertrophy (RVH) and right ventricular (RV) contractile dysfunction are major determinants of prognosis in pulmonary arterial hypertension (PAH) and PAH remains a severe disease. Recently, direct interruption of left ventricular hypertrophy has been suggested to decrease the risk of left-sided heart failure. Hexamethylene bis-acetamide inducible protein 1 (HEXIM1) is a negative regulator of positive transcription elongation factor b (P-TEFb), which activates RNA polymerase II (RNAPII)-dependent transcription and whose activation is strongly associated with left ventricular hypertrophy. We hypothesized that during the progression of PAH, increased P-TEFb activity might also play a role in RVH, and that HEXIM1 might have a preventive role against such process. We revealed that, in the mouse heart, HEXIM1 is highly expressed in the early postnatal period and its expression is gradually decreased, and that prostaglandin I(2), a therapeutic drug for PAH, increases HEXIM1 levels in cardiomyocytes. These results suggest that HEXIM1 might possess negative effect on cardiomyocyte growth and take part in cardiomyocyte regulation in RV. Using adenovirus-mediated gene delivery to cultured rat cardiomyocytes, we revealed that overexpression of HEXIM1 prevents endothelin-1-induced phosphorylation of RNAPII, cardiomyocyte hypertrophy, and mRNA expression of hypertrophic genes, whereas a HEXIM1 mutant lacking central basic region, which diminishes P-TEFb-suppressing activity, could not. Moreover, we created cardiomyocyte-specific HEXIM1 transgenic mice and revealed that HEXIM1 ameliorates RVH and prevents RV dilatation in hypoxia-induced PAH model. Taken together, these findings indicate that cardiomyocyte-specific overexpression of HEXIM1 inhibits progression to RVH under chronic hypoxia, most possibly via inhibition of P-TEFb-mediated enlargement of cardiomyocytes. We conclude that P-TEFb/HEXIM1-dependent transcriptional regulation may play a pathophysiological role in RVH and be a novel therapeutic target for mitigating RVH in PAH.
Conflict of interest statement
Figures

Similar articles
-
Down-regulation of hypoxia-inducible factor-1 alpha and vascular endothelial growth factor by HEXIM1 attenuates myocardial angiogenesis in hypoxic mice.Biochem Biophys Res Commun. 2014 Oct 24;453(3):600-5. doi: 10.1016/j.bbrc.2014.09.135. Epub 2014 Oct 6. Biochem Biophys Res Commun. 2014. PMID: 25301555
-
Interleukin-17 aggravates right ventricular remodeling via activating STAT3 under both normoxia and hypoxia.BMC Cardiovasc Disord. 2021 May 21;21(1):249. doi: 10.1186/s12872-021-02069-4. BMC Cardiovasc Disord. 2021. PMID: 34020615 Free PMC article.
-
Loss of KCNK3 is a hallmark of RV hypertrophy/dysfunction associated with pulmonary hypertension.Cardiovasc Res. 2018 May 1;114(6):880-893. doi: 10.1093/cvr/cvy016. Cardiovasc Res. 2018. PMID: 29360952
-
HEXIM1 and the control of transcription elongation: from cancer and inflammation to AIDS and cardiac hypertrophy.Cell Cycle. 2007 Aug 1;6(15):1856-63. doi: 10.4161/cc.6.15.4556. Epub 2007 Jun 6. Cell Cycle. 2007. PMID: 17671421 Review.
-
Brd4 and HEXIM1: multiple roles in P-TEFb regulation and cancer.Biomed Res Int. 2014;2014:232870. doi: 10.1155/2014/232870. Epub 2014 Jan 29. Biomed Res Int. 2014. PMID: 24592384 Free PMC article. Review.
Cited by
-
The crucial role of muscle glucocorticoid signaling in accelerating obesity and glucose intolerance via hyperinsulinemia.JCI Insight. 2023 Apr 24;8(8):e162382. doi: 10.1172/jci.insight.162382. JCI Insight. 2023. PMID: 36917179 Free PMC article.
-
HMBA ameliorates obesity by MYH9- and ACTG1-dependent regulation of hypothalamic neuropeptides.EMBO Mol Med. 2023 Dec 7;15(12):e18024. doi: 10.15252/emmm.202318024. Epub 2023 Nov 20. EMBO Mol Med. 2023. PMID: 37984341 Free PMC article.
-
P-TEFb as A Promising Therapeutic Target.Molecules. 2020 Feb 14;25(4):838. doi: 10.3390/molecules25040838. Molecules. 2020. PMID: 32075058 Free PMC article. Review.
-
RNA polymerase II transcription elongation control.Chem Rev. 2013 Nov 13;113(11):8583-603. doi: 10.1021/cr400105n. Epub 2013 Aug 6. Chem Rev. 2013. PMID: 23919563 Free PMC article. Review. No abstract available.
-
Gene-environment regulatory circuits of right ventricular pathology in tetralogy of fallot.J Mol Med (Berl). 2019 Dec;97(12):1711-1722. doi: 10.1007/s00109-019-01857-y. Epub 2019 Dec 13. J Mol Med (Berl). 2019. PMID: 31834445 Free PMC article.
References
-
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, et al. (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc. and the Pulmonary Hypertension Association. J Am Coll Cardiol 53: 1573–1619. - PubMed
-
- Michelakis ED, Wilkins MR, Rabinovitch M (2008) Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation 118: 1486–1495. - PubMed
-
- Haddad F, Doyle R, Murphy DJ, Hunt SA (2008) Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 117: 1717–1731. - PubMed
-
- Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF (2009) The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135: 794–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

