Quality control of photosystem II: lipid peroxidation accelerates photoinhibition under excessive illumination
- PMID: 23300595
- PMCID: PMC3531424
- DOI: 10.1371/journal.pone.0052100
Quality control of photosystem II: lipid peroxidation accelerates photoinhibition under excessive illumination
Erratum in
- PLoS One. 2013;8(5). doi:10.1371/annotation/8b0f8019-e4ba-4c35-9575-1a1313ae7b41
Abstract
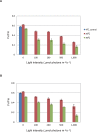
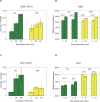
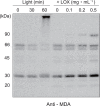
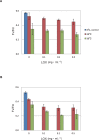
Environmental stresses lower the efficiency of photosynthesis and sometimes cause irreversible damage to plant functions. When spinach thylakoids and Photosystem II membranes were illuminated with excessive visible light (100-1,000 µmol photons m(-1) s(-1)) for 10 min at either 20°C or 30°C, the optimum quantum yield of Photosystem II decreased as the light intensity and temperature increased. Reactive oxygen species and endogenous cationic radicals produced through a photochemical reaction at and/or near the reaction center have been implicated in the damage to the D1 protein. Here we present evidence that lipid peroxidation induced by the illumination is involved in the damage to the D1 protein and the subunits of the light-harvesting complex of Photosystem II. This is reasoned from the results that considerable lipid peroxidation occurred in the thylakoids in the light, and that lipoxygenase externally added in the dark induced inhibition of Photosystem II activity in the thylakoids, production of singlet oxygen, which was monitored by electron paramagnetic resonance spin trapping, and damage to the D1 protein, in parallel with lipid peroxidation. Modification of the subunits of the light-harvesting complex of Photosystem II by malondialdehyde as well as oxidation of the subunits was also observed. We suggest that mainly singlet oxygen formed through lipid peroxidation under light stress participates in damaging the Photosystem II subunits.
Conflict of interest statement
Figures


Similar articles
-
Active oxygen produced during selective excitation of photosystem I is damaging not only to photosystem I, but also to photosystem II.Plant Physiol. 2001 Apr;125(4):2007-15. doi: 10.1104/pp.125.4.2007. Plant Physiol. 2001. PMID: 11299380 Free PMC article.
-
Quality control of photosystem II under light stress - turnover of aggregates of the D1 protein in vivo.Photosynth Res. 2005 Jun;84(1-3):29-33. doi: 10.1007/s11120-004-7310-7. Photosynth Res. 2005. PMID: 16049751
-
Quality control of photosystem II: reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress.J Biol Chem. 2008 Oct 17;283(42):28380-91. doi: 10.1074/jbc.M710465200. Epub 2008 Jul 29. J Biol Chem. 2008. PMID: 18664569 Free PMC article.
-
Quality control of PSII: behavior of PSII in the highly crowded grana thylakoids under excessive light.Plant Cell Physiol. 2014 Jul;55(7):1206-15. doi: 10.1093/pcp/pcu043. Epub 2014 Mar 7. Plant Cell Physiol. 2014. PMID: 24610582 Free PMC article. Review.
-
Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes.J Exp Bot. 2005 Jan;56(411):347-56. doi: 10.1093/jxb/eri041. Epub 2004 Nov 29. J Exp Bot. 2005. PMID: 15569703 Review.
Cited by
-
Quality control of Photosystem II: reversible and irreversible protein aggregation decides the fate of Photosystem II under excessive illumination.Front Plant Sci. 2013 Oct 29;4:433. doi: 10.3389/fpls.2013.00433. eCollection 2013. Front Plant Sci. 2013. PMID: 24194743 Free PMC article.
-
Transcriptome Analysis Revealed a Positive Role of Ethephon on Chlorophyll Metabolism of Zoysia japonica under Cold Stress.Plants (Basel). 2022 Feb 5;11(3):442. doi: 10.3390/plants11030442. Plants (Basel). 2022. PMID: 35161421 Free PMC article.
-
Singlet oxygen production by PSII under light stress: mechanism, detection and the protective role of β-carotene.Plant Cell Physiol. 2014 Jul;55(7):1216-23. doi: 10.1093/pcp/pcu040. Epub 2014 Feb 23. Plant Cell Physiol. 2014. PMID: 24566536 Free PMC article. Review.
-
Do motor plans affect sensorimotor state estimates during temporal decision-making with crossed vs. uncrossed hands? Failure to replicate the dynamic crossed-hand effect.Exp Brain Res. 2022 May;240(5):1529-1545. doi: 10.1007/s00221-022-06349-z. Epub 2022 Mar 24. Exp Brain Res. 2022. PMID: 35332358
-
Long-Chain Saturated Fatty Acids, Palmitic and Stearic Acids, Enhance the Repair of Photosystem II.Int J Mol Sci. 2020 Oct 12;21(20):7509. doi: 10.3390/ijms21207509. Int J Mol Sci. 2020. PMID: 33053769 Free PMC article.
References
-
- Barber J, Andersson B (1992) Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci 17: 61–66. - PubMed
-
- Aro EM, Virgin I, Andersson B (1993) Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134. - PubMed
-
- Yamamoto Y (2001) Quality control of photosystem II. Plant Cell Physiol 42: 121–128. - PubMed
-
- Yamamoto Y, Aminaka R, Yoshioka M, Khatoon M, Komayama K, et al. (2008) Quality control of photosystem II: impact of light and heat stresses. Photosynth Res 98: 589–608. - PubMed
-
- Macpherson AN, Telfer A, Barber J, Truscott TG (1993) Direct-Detection of Singlet Oxygen from Isolated Photosystem-II Reaction Centers. Biochimica Et Biophysica Acta 1143: 301–309.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

