Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs
- PMID: 23300456
- PMCID: PMC3536657
- DOI: 10.1371/journal.ppat.1003106
Recombinant HIV envelope proteins fail to engage germline versions of anti-CD4bs bNAbs
Erratum in
- PLoS Pathog. 2013 Jul;9(9). doi:10.1371/annotation/ed7c0148-97eb-4416-824d-6e6d1aaeceef
Abstract
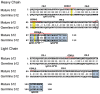
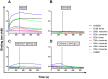
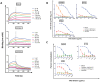
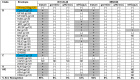
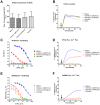
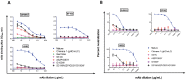
Vaccine candidates for HIV-1 so far have not been able to elicit broadly neutralizing antibodies (bNAbs) although they express the epitopes recognized by bNAbs to the HIV envelope glycoprotein (Env). To understand whether and how Env immunogens interact with the predicted germline versions of known bNAbs, we screened a large panel (N:56) of recombinant Envs (from clades A, B and C) for binding to the germline predecessors of the broadly neutralizing anti-CD4 binding site antibodies b12, NIH45-46 and 3BNC60. Although the mature antibodies reacted with diverse Envs, the corresponding germline antibodies did not display Env-reactivity. Experiments conducted with engineered chimeric antibodies combining the mature and germline heavy and light chains, respectively and vice-versa, revealed that both antibody chains are important for the known cross-reactivity of these antibodies. Our results also indicate that in order for b12 to display its broad cross-reactivity, multiple somatic mutations within its VH region are required. A consequence of the failure of the germline b12 to bind recombinant soluble Env is that Env-induced B-cell activation through the germline b12 BCR does not take place. Our study provides a new explanation for the difficulties in eliciting bNAbs with recombinant soluble Env immunogens. Our study also highlights the need for intense efforts to identify rare naturally occurring or engineered Envs that may engage the germline BCR versions of bNAbs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D.J Virol. 2014 Mar;88(5):2645-57. doi: 10.1128/JVI.03228-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352455 Free PMC article.
-
A single mutation turns a non-binding germline-like predecessor of broadly neutralizing antibody into a binding antibody to HIV-1 envelope glycoproteins.MAbs. 2011 Jul-Aug;3(4):402-7. doi: 10.4161/mabs.3.4.15740. Epub 2011 Jul 1. MAbs. 2011. PMID: 21540646 Free PMC article.
-
Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies.J Exp Med. 2013 Apr 8;210(4):655-63. doi: 10.1084/jem.20122824. Epub 2013 Mar 25. J Exp Med. 2013. PMID: 23530120 Free PMC article.
-
HIV Broadly Neutralizing Antibodies: VRC01 and Beyond.Adv Exp Med Biol. 2018;1075:53-72. doi: 10.1007/978-981-13-0484-2_3. Adv Exp Med Biol. 2018. PMID: 30030789 Review.
-
'Immunization during ART and ATI for HIV-1 vaccine discovery/development'.Curr Opin HIV AIDS. 2023 Nov 1;18(6):309-314. doi: 10.1097/COH.0000000000000817. Epub 2023 Sep 8. Curr Opin HIV AIDS. 2023. PMID: 37712859 Free PMC article. Review.
Cited by
-
Virological features associated with the development of broadly neutralizing antibodies to HIV-1.Trends Microbiol. 2015 Apr;23(4):204-11. doi: 10.1016/j.tim.2014.12.007. Epub 2015 Jan 5. Trends Microbiol. 2015. PMID: 25572881 Free PMC article. Review.
-
Priming HIV-1 broadly neutralizing antibody precursors in human Ig loci transgenic mice.Science. 2016 Sep 30;353(6307):1557-1560. doi: 10.1126/science.aah3945. Epub 2016 Sep 8. Science. 2016. PMID: 27608668 Free PMC article.
-
Deep Characterization of the Human Antibody Response to Natural Infection Using Longitudinal Immune Repertoire Sequencing.Mol Cell Proteomics. 2020 Feb;19(2):278-293. doi: 10.1074/mcp.RA119.001633. Epub 2019 Nov 25. Mol Cell Proteomics. 2020. PMID: 31767621 Free PMC article.
-
Structural insights on the role of antibodies in HIV-1 vaccine and therapy.Cell. 2014 Feb 13;156(4):633-48. doi: 10.1016/j.cell.2014.01.052. Cell. 2014. PMID: 24529371 Free PMC article. Review.
-
Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D.J Virol. 2014 Mar;88(5):2645-57. doi: 10.1128/JVI.03228-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352455 Free PMC article.
References
-
- Simek MD, Rida W, Priddy FH, Pung P, Carrow E, et al. (2009) Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. Journal of Virology 83: 7337–7348. - PMC - PubMed
-
- Burton DR, Pyati J, Koduri R, Sharp SJ, Thorton GB, et al. (1994) Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266: 1024–1027. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

