Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria
- PMID: 23300448
- PMCID: PMC3531520
- DOI: 10.1371/journal.ppat.1003099
Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria
Abstract
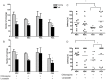
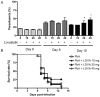
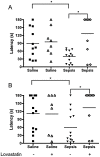
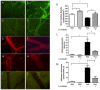
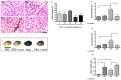
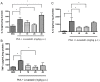
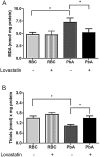
Cerebral malaria (CM) is the most severe manifestation of Plasmodium falciparum infection in children and non-immune adults. Previous work has documented a persistent cognitive impairment in children who survive an episode of CM that is mimicked in animal models of the disease. Potential therapeutic interventions for this complication have not been investigated, and are urgently needed. HMG-CoA reductase inhibitors (statins) are widely prescribed for cardiovascular diseases. In addition to their effects on the inhibition of cholesterol synthesis, statins have pleiotropic immunomodulatory activities. Here we tested if statins would prevent cognitive impairment in a murine model of cerebral malaria. Six days after infection with Plasmodium berghei ANKA (PbA) mice displayed clear signs of CM and were treated with chloroquine, or chloroquine and lovastatin. Intravital examination of pial vessels of infected animals demonstrated a decrease in functional capillary density and an increase in rolling and adhesion of leukocytes to inflamed endothelium that were reversed by treatment with lovastatin. In addition, oedema, ICAM-1, and CD11b mRNA levels were reduced in lovastatin-treated PbA-infected mice brains. Moreover, HMOX-1 mRNA levels are enhanced in lovastatin-treated healthy and infected brains. Oxidative stress and key inflammatory chemokines and cytokines were reduced to non-infected control levels in animals treated with lovastatin. Fifteen days post-infection cognitive dysfunction was detected by a battery of cognition tests in animals rescued from CM by chloroquine treatment. In contrast, it was absent in animals treated with lovastatin and chloroquine. The outcome was similar in experimental bacterial sepsis, suggesting that statins have neuroprotective effects in severe infectious syndromes in addition to CM. Statin treatment prevents neuroinflammation and blood brain barrier dysfunction in experimental CM and related conditions that are associated with cognitive sequelae, and may be a valuable adjuvant therapeutic agent for prevention of cognitive impairment in patients surviving an episode of CM.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy.PLoS Pathog. 2010 Jun 24;6(6):e1000963. doi: 10.1371/journal.ppat.1000963. PLoS Pathog. 2010. PMID: 20585569 Free PMC article.
-
Vascular endothelial growth factor (VEGF) and lovastatin suppress the inflammatory response to Plasmodium berghei infection and protect against experimental cerebral malaria.Pathog Glob Health. 2015 Sep;109(6):266-74. doi: 10.1179/2047773215Y.0000000021. Epub 2015 Sep 21. Pathog Glob Health. 2015. PMID: 26392164 Free PMC article.
-
Exogenous nitric oxide decreases brain vascular inflammation, leakage and venular resistance during Plasmodium berghei ANKA infection in mice.J Neuroinflammation. 2011 Jun 7;8:66. doi: 10.1186/1742-2094-8-66. J Neuroinflammation. 2011. PMID: 21649904 Free PMC article.
-
Genetic analysis of cerebral malaria in the mouse model infected with Plasmodium berghei.Mamm Genome. 2018 Aug;29(7-8):488-506. doi: 10.1007/s00335-018-9752-9. Epub 2018 Jun 19. Mamm Genome. 2018. PMID: 29922917 Review.
-
Statins and the vascular endothelial inflammatory response.Trends Immunol. 2007 Feb;28(2):88-98. doi: 10.1016/j.it.2006.12.003. Epub 2007 Jan 2. Trends Immunol. 2007. PMID: 17197237 Free PMC article. Review.
Cited by
-
Interactions between Autophagy and Bacterial Toxins: Targets for Therapy?Toxins (Basel). 2015 Aug 4;7(8):2918-58. doi: 10.3390/toxins7082918. Toxins (Basel). 2015. PMID: 26248079 Free PMC article. Review.
-
Melatonin signaling and its modulation of PfNF-YB transcription factor expression in Plasmodium falciparum.Int J Mol Sci. 2013 Jul 1;14(7):13704-18. doi: 10.3390/ijms140713704. Int J Mol Sci. 2013. PMID: 23839089 Free PMC article. Review.
-
Zika Virus Infects, Activates, and Crosses Brain Microvascular Endothelial Cells, without Barrier Disruption.Front Microbiol. 2017 Dec 22;8:2557. doi: 10.3389/fmicb.2017.02557. eCollection 2017. Front Microbiol. 2017. PMID: 29312238 Free PMC article.
-
Further evidence for an anti-inflammatory role of artesunate in experimental cerebral malaria.Malar J. 2013 Nov 2;12:388. doi: 10.1186/1475-2875-12-388. Malar J. 2013. PMID: 24180288 Free PMC article.
-
Targeting the Blood-Brain Barrier to Prevent Sepsis-Associated Cognitive Impairment.J Cent Nerv Syst Dis. 2019 Apr 9;11:1179573519840652. doi: 10.1177/1179573519840652. eCollection 2019. J Cent Nerv Syst Dis. 2019. PMID: 31007531 Free PMC article. Review.
References
-
- Kappe SH, Vaughan AM, Boddey JA, Cowman AF (2010) That was then but this is now: malaria research in the time of an eradication agenda. Science 328: 862–866. - PubMed
-
- Rosenthal PJ (2008) Artesunate for the treatment of severe falciparum malaria. N Engl J Med 358: 1829–1836. - PubMed
-
- de Souza JB, Hafalla JC, Riley EM, Couper KN (2010) Cerebral malaria: why experimental murine models are required to understand the pathogenesis of disease. Parasitology 137: 755–772. - PubMed
-
- Golenser J, McQuillan J, Hee L, Mitchell AJ, Hunt NH (2006) Conventional and experimental treatment of cerebral malaria. Int J Parasitol 36: 583–593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

