Intracortical circuits, sensorimotor integration and plasticity in human motor cortical projections to muscles of the lower face
- PMID: 23297305
- PMCID: PMC3624858
- DOI: 10.1113/jphysiol.2012.245746
Intracortical circuits, sensorimotor integration and plasticity in human motor cortical projections to muscles of the lower face
Abstract
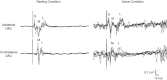
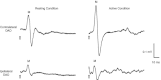
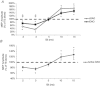
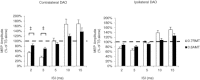
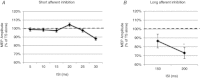
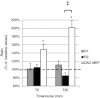
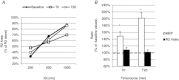
Previous studies of the cortical control of human facial muscles documented the distribution of corticobulbar projections and the presence of intracortical inhibitory and facilitatory mechanisms. Yet surprisingly, given the importance and precision in control of facial expression, there have been no studies of the afferent modulation of corticobulbar excitability or of the plasticity of synaptic connections in the facial primary motor cortex (face M1). In 25 healthy volunteers, we used standard single- and paired-pulse transcranial magnetic stimulation (TMS) methods to probe motor-evoked potentials (MEPs), short-intracortical inhibition, intracortical facilitation, short-afferent and long-afferent inhibition and paired associative stimulation in relaxed and active depressor anguli oris muscles. Single-pulse TMS evoked bilateral MEPs at rest and during activity that were larger in contralateral muscles, confirming that corticobulbar projection to lower facial muscles is bilateral and asymmetric, with contralateral predominance. Both short-intracortical inhibition and intracortical facilitation were present bilaterally in resting and active conditions. Electrical stimulation of the facial nerve paired with a TMS pulse 5-200 ms later showed no short-afferent inhibition, but long-afferent inhibition was present. Paired associative stimulation tested with an electrical stimulation-TMS interval of 20 ms significantly facilitated MEPs for up to 30 min. The long-term potentiation, evoked for the first time in face M1, demonstrates that excitability of the facial motor cortex is prone to plastic changes after paired associative stimulation. Evaluation of intracortical circuits in both relaxed and active lower facial muscles as well as of plasticity in the facial motor cortex may provide further physiological insight into pathologies affecting the facial motor system.
Figures



Similar articles
-
Role of cutaneous and proprioceptive inputs in sensorimotor integration and plasticity occurring in the facial primary motor cortex.J Physiol. 2020 Feb;598(4):839-851. doi: 10.1113/JP278877. Epub 2020 Feb 3. J Physiol. 2020. PMID: 31876950
-
Is it possible to compare inhibitory and excitatory intracortical circuits in face and hand primary motor cortex?J Physiol. 2022 Aug;600(15):3567-3583. doi: 10.1113/JP283137. Epub 2022 Jul 14. J Physiol. 2022. PMID: 35801987 Free PMC article.
-
Representation of facial muscles in human motor cortex.J Physiol. 2005 Aug 15;567(Pt 1):323-36. doi: 10.1113/jphysiol.2005.088542. Epub 2005 Jun 9. J Physiol. 2005. PMID: 15946959 Free PMC article.
-
Pharmaco-transcranial magnetic stimulation studies of motor excitability.Handb Clin Neurol. 2013;116:387-97. doi: 10.1016/B978-0-444-53497-2.00032-2. Handb Clin Neurol. 2013. PMID: 24112911 Review.
-
Exploring the connections between basal ganglia and cortex revealed by transcranial magnetic stimulation, evoked potential and deep brain stimulation in dystonia.Eur J Paediatr Neurol. 2022 Jan;36:69-77. doi: 10.1016/j.ejpn.2021.12.004. Epub 2021 Dec 10. Eur J Paediatr Neurol. 2022. PMID: 34922163 Review.
Cited by
-
Invasive versus non-invasive mapping of the motor cortex.Hum Brain Mapp. 2020 Oct 1;41(14):3970-3983. doi: 10.1002/hbm.25101. Epub 2020 Jun 26. Hum Brain Mapp. 2020. PMID: 32588936 Free PMC article.
-
Application of facial neuromuscular electrical stimulation (fNMES) in psychophysiological research: Practical recommendations based on a systematic review of the literature.Behav Res Methods. 2024 Apr;56(4):2941-2976. doi: 10.3758/s13428-023-02262-7. Epub 2023 Oct 20. Behav Res Methods. 2024. PMID: 37864116 Free PMC article.
-
Trigeminal nerve stimulation modulates brainstem more than cortical excitability in healthy humans.Exp Brain Res. 2015 Nov;233(11):3301-11. doi: 10.1007/s00221-015-4398-2. Epub 2015 Aug 11. Exp Brain Res. 2015. PMID: 26259748
-
Volunteers' concerns about facial neuromuscular electrical stimulation.BMC Psychol. 2022 May 7;10(1):117. doi: 10.1186/s40359-022-00827-3. BMC Psychol. 2022. PMID: 35526073 Free PMC article.
-
Happy faces selectively increase the excitability of cortical neurons innervating frowning muscles of the mouth.Exp Brain Res. 2020 Apr;238(4):1043-1049. doi: 10.1007/s00221-020-05777-z. Epub 2020 Mar 21. Exp Brain Res. 2020. PMID: 32200403
References
-
- Baad-Hansen L, Blicher JU, Lapitskaya N, Nielsen JF, Svensson P. Intra-cortical excitability in healthy human subjects after tongue training. J Oral Rehabil. 2009;36:427–434. - PubMed
-
- Benecke R, Meyer BU, Schönle P, Conrad B. Transcranial magnetic stimulation of the human brain: responses in muscles supplied by cranial nerves. Exp Brain Res. 1988;71:623–632. - PubMed
-
- Bennett AJ, Wastell DG, Barker GR, Blackburn CW, Rood JP. Trigeminal somatosensory evoked potentials. A review of the literature as applicable to oral dysaesthesias. Int J Oral Maxillofac Surg. 1987;16:408–415. - PubMed
-
- Bikmullina R, Bäumer T, Zittel S, Münchau A. Sensory afferent inhibition within and between limbs in humans. Clin Neurophysiol. 2009;120:610–618. - PubMed
-
- Bologna M, Agostino R, Gregori B, Belvisi D, Manfredi M, Berardelli A. Metaplasticity of the human trigeminal blink reflex. Eur J Neurosci. 2010;32:1707–1714. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

