Robust T-cell stimulation by Epstein-Barr virus-transformed B cells after antigen targeting to DEC-205
- PMID: 23297134
- PMCID: PMC3587321
- DOI: 10.1182/blood-2012-08-450775
Robust T-cell stimulation by Epstein-Barr virus-transformed B cells after antigen targeting to DEC-205
Abstract
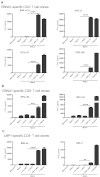
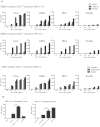
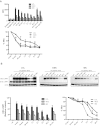
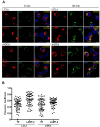
DEC-205 is a type I transmembrane multilectin receptor that is predominantly expressed on dendritic cells (DCs). Therefore, previous studies primarily focused on processing of DEC-205–targeted antigens by this potent antigen presenting cell type. Here we show that Epstein-Barr virus (EBV) transformed lymphoblastoid B-cell lines (LCLs) not only express DEC-205 at similar levels to DCs, but also efficiently present targeted EBV nuclear antigen 1 (EBNA1) and EBV-latent membrane protein 1 (LMP1) to EBNA1- and LMP1-specific CD4+ and CD8+ T-cell clones in vitro. Targeting of antigens to DEC-205 on B cells led to more efficient MHC class II than I loading, and stimulated T cells more efficiently than targeting to DEC-205 on DCs. Although LCLs internalized DEC-205–targeted antigens less efficiently than DCs, they retained them for longer time periods and delivered them to endosomal compartments that receive also B-cell receptor targeted proteins. This could facilitate prolonged T-cell stimulation and efficient MHC class II loading, and, indeed, CD4+ T-cell expansion by DEC-205–targeted vaccination was significantly compromised in B-cell deficient mice. These studies suggest that B cells, activated by virus transformation or other means, can contribute to T-cell stimulation after DEC-205 targeting of antigens during vaccination.
Figures

Similar articles
-
Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses.Blood. 2008 Aug 15;112(4):1231-9. doi: 10.1182/blood-2008-03-148072. Epub 2008 Jun 2. Blood. 2008. PMID: 18519810 Free PMC article.
-
Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation.Blood. 2010 Sep 30;116(13):2277-85. doi: 10.1182/blood-2010-02-268425. Epub 2010 Jun 21. Blood. 2010. PMID: 20566893
-
Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1.J Exp Med. 2000 May 15;191(10):1649-60. doi: 10.1084/jem.191.10.1649. J Exp Med. 2000. PMID: 10811859 Free PMC article.
-
Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity.J Intern Med. 2012 Feb;271(2):183-92. doi: 10.1111/j.1365-2796.2011.02496.x. Epub 2012 Jan 4. J Intern Med. 2012. PMID: 22126373 Free PMC article. Review.
-
LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination.Trends Immunol. 2003 Dec;24(12):619-22. doi: 10.1016/j.it.2003.10.001. Trends Immunol. 2003. PMID: 14644131 Review.
Cited by
-
Altered Immune Response to the Epstein-Barr Virus as a Prerequisite for Multiple Sclerosis.Cells. 2022 Sep 4;11(17):2757. doi: 10.3390/cells11172757. Cells. 2022. PMID: 36078165 Free PMC article. Review.
-
CD141+ dendritic cells produce prominent amounts of IFN-α after dsRNA recognition and can be targeted via DEC-205 in humanized mice.Blood. 2013 Jun 20;121(25):5034-44. doi: 10.1182/blood-2012-12-473413. Epub 2013 Mar 12. Blood. 2013. PMID: 23482932 Free PMC article.
-
Plasmacytoid dendritic cells respond to Epstein-Barr virus infection with a distinct type I interferon subtype profile.Blood Adv. 2019 Apr 9;3(7):1129-1144. doi: 10.1182/bloodadvances.2018025536. Blood Adv. 2019. PMID: 30952679 Free PMC article.
-
Infection and immune control of human oncogenic γ-herpesviruses in humanized mice.Philos Trans R Soc Lond B Biol Sci. 2019 May 27;374(1773):20180296. doi: 10.1098/rstb.2018.0296. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30955487 Free PMC article. Review.
-
Epstein Barr Virus Exploits Genetic Susceptibility to Increase Multiple Sclerosis Risk.Microorganisms. 2021 Oct 21;9(11):2191. doi: 10.3390/microorganisms9112191. Microorganisms. 2021. PMID: 34835317 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

