The tension mounts: stress fibers as force-generating mechanotransducers
- PMID: 23295347
- PMCID: PMC3542796
- DOI: 10.1083/jcb.201210090
The tension mounts: stress fibers as force-generating mechanotransducers
Abstract
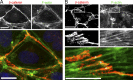
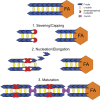
Stress fibers (SFs) are often the most prominent cytoskeletal structures in cells growing in tissue culture. Composed of actin filaments, myosin II, and many other proteins, SFs are force-generating and tension-bearing structures that respond to the surrounding physical environment. New work is shedding light on the mechanosensitive properties of SFs, including that these structures can respond to mechanical tension by rapid reinforcement and that there are mechanisms to repair strain-induced damage. Although SFs are superficially similar in organization to the sarcomeres of striated muscle, there are intriguing differences in their organization and behavior, indicating that much still needs to be learned about these structures.
Figures


Similar articles
-
What factors determine the number of nonmuscle myosin II in the sarcomeric unit of stress fibers?Biomech Model Mechanobiol. 2021 Feb;20(1):155-166. doi: 10.1007/s10237-020-01375-8. Epub 2020 Aug 10. Biomech Model Mechanobiol. 2021. PMID: 32776260
-
Estimation of the mechanical connection between apical stress fibers and the nucleus in vascular smooth muscle cells cultured on a substrate.J Biomech. 2014 Apr 11;47(6):1422-9. doi: 10.1016/j.jbiomech.2014.01.042. Epub 2014 Jan 31. J Biomech. 2014. PMID: 24548337
-
Asymmetric response emerges between creation and disintegration of force-bearing subcellular structures as revealed by percolation analysis.Integr Biol (Camb). 2024 Jan 23;16:zyae012. doi: 10.1093/intbio/zyae012. Integr Biol (Camb). 2024. PMID: 38900169
-
Caveolae - mechanosensitive membrane invaginations linked to actin filaments.J Cell Sci. 2015 Aug 1;128(15):2747-58. doi: 10.1242/jcs.153940. Epub 2015 Jul 9. J Cell Sci. 2015. PMID: 26159735 Review.
-
The inner workings of stress fibers - from contractile machinery to focal adhesions and back.J Cell Sci. 2016 Apr 1;129(7):1293-304. doi: 10.1242/jcs.180927. J Cell Sci. 2016. PMID: 27037413 Review.
Cited by
-
Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function.Int J Mol Sci. 2021 Mar 23;22(6):3279. doi: 10.3390/ijms22063279. Int J Mol Sci. 2021. PMID: 33807043 Free PMC article. Review.
-
Spectrin regulates cell contractility through production and maintenance of actin bundles in the Caenorhabditis elegans spermatheca.Mol Biol Cell. 2018 Oct 1;29(20):2433-2449. doi: 10.1091/mbc.E18-06-0347. Epub 2018 Aug 9. Mol Biol Cell. 2018. PMID: 30091661 Free PMC article.
-
Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA.J Cell Sci. 2017 Mar 1;130(5):892-902. doi: 10.1242/jcs.196881. Epub 2017 Jan 17. J Cell Sci. 2017. PMID: 28096473 Free PMC article.
-
Centrifugal Displacement of Nuclei Reveals Multiple LINC Complex Mechanisms for Homeostatic Nuclear Positioning.Curr Biol. 2017 Oct 23;27(20):3097-3110.e5. doi: 10.1016/j.cub.2017.08.073. Epub 2017 Oct 5. Curr Biol. 2017. PMID: 28988861 Free PMC article.
-
The toxoplasma-host cell junction is anchored to the cell cortex to sustain parasite invasive force.BMC Biol. 2014 Dec 31;12:773. doi: 10.1186/s12915-014-0108-y. BMC Biol. 2014. PMID: 25551479 Free PMC article.
References
-
- Adamson P., Etienne S., Couraud P.O., Calder V., Greenwood J. 1999. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J. Immunol. 162:2964–2973 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

