The induction of stromule formation by a plant DNA-virus in epidermal leaf tissues suggests a novel intra- and intercellular macromolecular trafficking route
- PMID: 23293643
- PMCID: PMC3530832
- DOI: 10.3389/fpls.2012.00291
The induction of stromule formation by a plant DNA-virus in epidermal leaf tissues suggests a novel intra- and intercellular macromolecular trafficking route
Abstract
Stromules are dynamic thin protrusions of membrane envelope from plant cell plastids. Despite considerable progress in understanding the importance of certain cytoskeleton elements and motor proteins for stromule maintenance, their function within the cell has yet to be unraveled. Several viruses cause a remodulation of plastid structures and stromule biogenesis within their host plants. For RNA-viruses these interactions were demonstrated to be relevant to the infection process. An involvement of plastids and stromules is assumed in the DNA-virus life cycle as well, but their functional role needs to be determined. Recent findings support a participation of heat shock cognate 70 kDa protein (cpHSC70-1)-containing stromules induced by a DNA-virus infection (Abutilon mosaic virus, AbMV, Geminiviridae) in intra- and intercellular molecule exchange. The chaperone cpHSC70-1 was shown to interact with the AbMV movement protein (MP). Bimolecular fluorescence complementation confirmed the interaction of cpHSC70-1 and MP, and showed a homo-oligomerization of either protein in planta. The complexes were detected at the cellular margin and co-localized with plastids. In healthy plant tissues cpHSC70-1-oligomers occurred in distinct spots at chloroplasts and in small filaments extending from plastids to the cell periphery. AbMV-infection induced a cpHSC70-1-containing stromule network that exhibits elliptical dilations and transverses whole cells. Silencing of the cpHSC70 gene revealed an impact of cpHSC70 on chloroplast stability and restricted AbMV movement, but not viral DNA accumulation. Based on these data, a model is suggested in which these stromules function in molecule exchange between plastids and other organelles and perhaps other cells. AbMV may utilize cpHSC70-1 for trafficking along plastids and stromules into a neighboring cell or from plastids into the nucleus. Experimental approaches to investigate this hypothesis are discussed.
Keywords: chaperone; geminivirus; heat shock protein; movement protein; plastid.
Figures
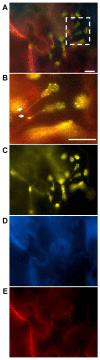
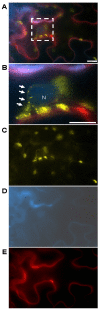

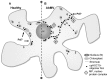
Similar articles
-
A plastid-targeted heat shock cognate 70kDa protein interacts with the Abutilon mosaic virus movement protein.Virology. 2010 May 25;401(1):6-17. doi: 10.1016/j.virol.2010.02.011. Epub 2010 Mar 2. Virology. 2010. PMID: 20193958
-
Induction of stromule formation by extracellular sucrose and glucose in epidermal leaf tissue of Arabidopsis thaliana.BMC Plant Biol. 2011 Aug 16;11:115. doi: 10.1186/1471-2229-11-115. BMC Plant Biol. 2011. PMID: 21846357 Free PMC article.
-
Begomoviral Movement Protein Effects in Human and Plant Cells: Towards New Potential Interaction Partners.Viruses. 2017 Nov 9;9(11):334. doi: 10.3390/v9110334. Viruses. 2017. PMID: 29120369 Free PMC article.
-
Stromules, functional extensions of plastids within the plant cell.Curr Opin Plant Biol. 2020 Dec;58:25-32. doi: 10.1016/j.pbi.2020.10.005. Epub 2020 Nov 1. Curr Opin Plant Biol. 2020. PMID: 33137706 Review.
-
Stromules and the dynamic nature of plastid morphology.J Microsc. 2004 May;214(Pt 2):124-37. doi: 10.1111/j.0022-2720.2004.01317.x. J Microsc. 2004. PMID: 15102061 Review.
Cited by
-
Chloroplast Stromules Function during Innate Immunity.Dev Cell. 2015 Jul 6;34(1):45-57. doi: 10.1016/j.devcel.2015.05.011. Epub 2015 Jun 25. Dev Cell. 2015. PMID: 26120031 Free PMC article.
-
Chloroplast in Plant-Virus Interaction.Front Microbiol. 2016 Oct 4;7:1565. doi: 10.3389/fmicb.2016.01565. eCollection 2016. Front Microbiol. 2016. PMID: 27757106 Free PMC article. Review.
-
Begomovirus-Host Interactions: Viral Proteins Orchestrating Intra and Intercellular Transport of Viral DNA While Suppressing Host Defense Mechanisms.Viruses. 2023 Jul 21;15(7):1593. doi: 10.3390/v15071593. Viruses. 2023. PMID: 37515277 Free PMC article. Review.
-
The Formation of Stromules In Vitro from Chloroplasts Isolated from Nicotiana benthamiana.PLoS One. 2016 Feb 3;11(2):e0146489. doi: 10.1371/journal.pone.0146489. eCollection 2016. PLoS One. 2016. PMID: 26840974 Free PMC article.
-
Effector XopQ-induced stromule formation in Nicotiana benthamiana depends on ETI signaling components ADR1 and NRG1.Plant Physiol. 2023 Jan 2;191(1):161-176. doi: 10.1093/plphys/kiac481. Plant Physiol. 2023. PMID: 36259930 Free PMC article.
References
-
- Aberle H. J., Rütz M. L., Karayavuz M., Frischmuth S., Wege C., Hülser D., et al. (2002). Localizing BC1 movement proteins of Abutilon mosaic geminivirus in yeasts by subcellular fractionation and freeze-fracture immunolabelling. Arch. Virol. 147 103–107 - PubMed
LinkOut - more resources
Full Text Sources

