Regulation of erythropoiesis by hypoxia-inducible factors
- PMID: 23291219
- PMCID: PMC3731139
- DOI: 10.1016/j.blre.2012.12.003
Regulation of erythropoiesis by hypoxia-inducible factors
Abstract
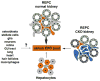
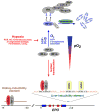
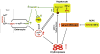
A classic physiologic response to systemic hypoxia is the increase in red blood cell production. Hypoxia-inducible factors (HIFs) orchestrate this response by inducing cell-type specific gene expression changes that result in increased erythropoietin (EPO) production in kidney and liver, in enhanced iron uptake and utilization and in adjustments of the bone marrow microenvironment that facilitate erythroid progenitor maturation and proliferation. In particular HIF-2 has emerged as the transcription factor that regulates EPO synthesis in the kidney and liver and plays a critical role in the regulation of intestinal iron uptake. Its key function in the hypoxic regulation of erythropoiesis is underscored by genetic studies in human populations that live at high-altitude and by mutational analysis of patients with familial erythrocytosis. This review provides a perspective on recent insights into HIF-controlled erythropoiesis and iron metabolism, and examines cell types that have EPO-producing capability. Furthermore, the review summarizes clinical syndromes associated with mutations in the O(2)-sensing pathway and the genetic changes that occur in high altitude natives. The therapeutic potential of pharmacologic HIF activation for the treatment of anemia is discussed.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The author serves on the Scientific Advisory Board of Akebia Therapeutics, a company that develops prolyl-4-hydroxylase inhibitors for the treatment of anemia.
Figures
Similar articles
-
Hypoxic regulation of erythropoiesis and iron metabolism.Am J Physiol Renal Physiol. 2010 Jul;299(1):F1-13. doi: 10.1152/ajprenal.00174.2010. Epub 2010 May 5. Am J Physiol Renal Physiol. 2010. PMID: 20444740 Free PMC article. Review.
-
Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia.Blood. 2010 Oct 21;116(16):3039-48. doi: 10.1182/blood-2010-02-270322. Epub 2010 Jul 13. Blood. 2010. PMID: 20628150 Free PMC article.
-
Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis.J Clin Invest. 2012 Dec;122(12):4635-44. doi: 10.1172/JCI63924. Epub 2012 Nov 1. J Clin Invest. 2012. PMID: 23114598 Free PMC article.
-
Oxygen-regulated expression of the erythropoietin gene in the human renal cell line REPC.Blood. 2011 May 5;117(18):4905-14. doi: 10.1182/blood-2010-07-298083. Epub 2011 Mar 15. Blood. 2011. Retraction in: Blood. 2014 May 22;123(21):3365. doi: 10.1182/blood-2014-04-571752 PMID: 21406725 Retracted.
-
Hypoxia Pathway Proteins are Master Regulators of Erythropoiesis.Int J Mol Sci. 2020 Oct 30;21(21):8131. doi: 10.3390/ijms21218131. Int J Mol Sci. 2020. PMID: 33143240 Free PMC article. Review.
Cited by
-
Pathology-supported genetic testing as a method for disability prevention in multiple sclerosis (MS). Part II. Insights from two MS cases.Metab Brain Dis. 2021 Aug;36(6):1169-1181. doi: 10.1007/s11011-021-00712-9. Epub 2021 Mar 12. Metab Brain Dis. 2021. PMID: 33710528 Review.
-
Bidirectional signalling between EphA2 and ephrinA1 increases tubular cell attachment, laminin secretion and modulates erythropoietin expression after renal hypoxic injury.Pflugers Arch. 2016 Aug;468(8):1433-48. doi: 10.1007/s00424-016-1838-1. Epub 2016 May 26. Pflugers Arch. 2016. PMID: 27228995
-
Neuroprotective Effect of HIF Prolyl Hydroxylase Inhibition in an In Vitro Hypoxia Model.Antioxidants (Basel). 2020 Jul 24;9(8):662. doi: 10.3390/antiox9080662. Antioxidants (Basel). 2020. PMID: 32722310 Free PMC article.
-
Hepcidin and erythroferrone response to 3 weeks of exposure to normobaric hypoxia at rest in trained cyclists.Front Physiol. 2023 Nov 27;14:1279827. doi: 10.3389/fphys.2023.1279827. eCollection 2023. Front Physiol. 2023. PMID: 38089475 Free PMC article.
-
Cloning of the Human MORG1 Promoter: Differential Regulation by Hypoxia and Prolyl-Hydroxylase Inhibitors.Genes (Basel). 2022 Feb 25;13(3):427. doi: 10.3390/genes13030427. Genes (Basel). 2022. PMID: 35327980 Free PMC article.
References
-
- Bert P. Sur la richesse en hemoglobine du sang des animaux vivant sur les hauts lieux. C R Acad Sci Paris. 1882;94:805–7.
-
- Jourdanet D. Influence de la pression de l’air sur la vie de l’homme. Paris: G Masson; 1875.
-
- Bert P. Recherches de physiologie expérimentale. Paris: G Masson; 1878. La pression barométrique.
-
- Viault F. Sur laugmentation considérable du nombre des globules rouges dans le sang chez les inhabitants des hauts plateaux de l’Amérique du Sud. C R Acad Sci Paris. 1890;111:917–8.
-
- Jacobson LO, Goldwasser E, Fried W, Plzak L. Role of the kidney in erythropoiesis. Nature. 1957;179:633–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

