Pericytes from infantile hemangioma display proangiogenic properties and dysregulated angiopoietin-1
- PMID: 23288163
- PMCID: PMC3573237
- DOI: 10.1161/ATVBAHA.112.300929
Pericytes from infantile hemangioma display proangiogenic properties and dysregulated angiopoietin-1
Abstract
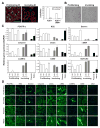
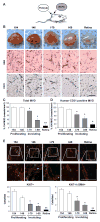
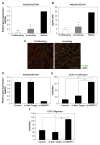
Objective: Infantile hemangioma (IH) is a rapidly growing vascular tumor affecting newborns. It is composed of immature endothelial cells and pericytes that proliferate into a disorganized mass of blood vessels. We isolated pericytes from IH (Hem-pericytes) to test our hypothesis that Hem-pericytes are unable to stabilize blood vessels.
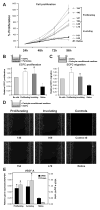
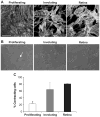
Methods and results: We injected pericytes in vivo, in combination with endothelial cells, and found that Hem-pericytes formed more microvessels compared with control retinal pericytes. We, thereby, analyzed proangiogenic properties of the Hem-pericytes. They grew fast in vitro, and were unable to stabilize endothelial cell growth and migration, and expressed high levels of vascular endothelial growth factor-A compared with retinal pericytes. Hem-pericytes from proliferating phase IH showed lower contractility in vitro, compared with Hem-pericytes from the involuting phase and retinal pericytes. Consistent with a diminished ability to stabilize endothelium, angiopoietin 1 was reduced in Hem-pericytes compared with retinal pericytes. Normal retinal pericytes in which angiopoietin 1 was silenced produced conditioned medium that stimulated endothelial cell proliferation and migration.
Conclusions: We report the first successful isolation of patient-derived pericytes from IH tissue. Hem-pericytes exhibited proangiogenic properties and low levels of angiopoietin 1, consistent with a diminished ability to stabilize blood vessels in IH.
Figures
Similar articles
-
JAGGED1 signaling regulates hemangioma stem cell-to-pericyte/vascular smooth muscle cell differentiation.Arterioscler Thromb Vasc Biol. 2011 Oct;31(10):2181-92. doi: 10.1161/ATVBAHA.111.232934. Epub 2011 Jul 14. Arterioscler Thromb Vasc Biol. 2011. PMID: 21757656 Free PMC article.
-
Jagged1/Notch3 Signaling Modulates Hemangioma-Derived Pericyte Proliferation and Maturation.Cell Physiol Biochem. 2016;40(5):895-907. doi: 10.1159/000453148. Epub 2016 Dec 7. Cell Physiol Biochem. 2016. PMID: 27941324
-
Fibulin-7 is overexpressed in glioblastomas and modulates glioblastoma neovascularization through interaction with angiopoietin-1.Int J Cancer. 2019 Oct 15;145(8):2157-2169. doi: 10.1002/ijc.32306. Epub 2019 Apr 15. Int J Cancer. 2019. PMID: 30924128
-
Recent advances in understanding the molecular basis of infantile haemangioma development.Br J Dermatol. 2024 Oct 17;191(5):661-669. doi: 10.1093/bjd/ljae241. Br J Dermatol. 2024. PMID: 38845569 Review.
-
Insights into the mechanisms of angiogenesis in infantile hemangioma.Biomed Pharmacother. 2024 Sep;178:117181. doi: 10.1016/j.biopha.2024.117181. Epub 2024 Jul 25. Biomed Pharmacother. 2024. PMID: 39059349 Review.
Cited by
-
Propranolol for the treatment of vascular sarcomas.J Exp Pharmacol. 2018 Sep 6;10:51-58. doi: 10.2147/JEP.S146211. eCollection 2018. J Exp Pharmacol. 2018. PMID: 30233257 Free PMC article. Review.
-
Combinative effects of β-elemene and propranolol on the proliferation, migration, and angiogenesis of hemangioma.PeerJ. 2023 Jul 12;11:e15643. doi: 10.7717/peerj.15643. eCollection 2023. PeerJ. 2023. PMID: 37456875 Free PMC article.
-
Infantile hemangioma: the common and enigmatic vascular tumor.J Clin Invest. 2024 Apr 15;134(8):e172836. doi: 10.1172/JCI172836. J Clin Invest. 2024. PMID: 38618963 Free PMC article. Review.
-
Global research trends of infantile hemangioma: A bibliometric and visualization analysis from 2000 to 2022.Heliyon. 2023 Oct 21;9(11):e21300. doi: 10.1016/j.heliyon.2023.e21300. eCollection 2023 Nov. Heliyon. 2023. PMID: 37920523 Free PMC article.
-
Propranolol enhanced adipogenesis instead of induction of apoptosis of hemangiomas stem cells.Int J Clin Exp Pathol. 2014 Jun 15;7(7):3809-17. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25120757 Free PMC article.
References
-
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

