Notch1 is not required for acinar-to-ductal metaplasia in a model of Kras-induced pancreatic ductal adenocarcinoma
- PMID: 23284900
- PMCID: PMC3526595
- DOI: 10.1371/journal.pone.0052133
Notch1 is not required for acinar-to-ductal metaplasia in a model of Kras-induced pancreatic ductal adenocarcinoma
Abstract
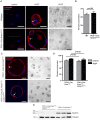
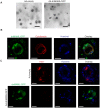
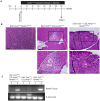
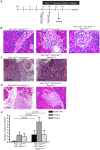
Pancreatic ductal adenocarcinoma is believed to arise from precursor lesions termed pancreatic intraepithelial neoplasia (PanIN). Mouse models have demonstrated that targeted expression of activated K-ras to mature acinar cells in the pancreas induces the spontaneous development of PanIN lesions; implying acinar-to-ductal metaplasia (ADM) is a key event in this process. Recent studies suggest Notch signaling is a key regulator of ADM. To assess if Notch1 is required for K-ras driven ADM we employed both an in vivo mouse model and in vitro explant culture system, in which an oncogenic allele of K-ras is activated and Notch1 is deleted simultaneously in acinar cells. Our results demonstrate that oncogenic K-ras is sufficient to drive ADM both in vitro and in vivo but that loss of Notch1 has a minimal effect on this process. Interestingly, while loss of Notch1 in vivo does not affect the severity of PanIN lesions observed, the overall numbers of lesions were greater in mice with deleted Notch1. This suggests Notch1 deletion renders acinar cells more susceptible to formation of K-ras-induced PanINs.
Conflict of interest statement
Figures
Similar articles
-
Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice.Gastroenterology. 2018 Apr;154(5):1494-1508.e13. doi: 10.1053/j.gastro.2017.12.005. Epub 2017 Dec 15. Gastroenterology. 2018. PMID: 29248441 Free PMC article.
-
Maintenance of acinar cell organization is critical to preventing Kras-induced acinar-ductal metaplasia.Oncogene. 2013 Apr 11;32(15):1950-8. doi: 10.1038/onc.2012.210. Epub 2012 Jun 4. Oncogene. 2013. PMID: 22665051 Free PMC article.
-
PYK2 Is Involved in Premalignant Acinar Cell Reprogramming and Pancreatic Ductal Adenocarcinoma Maintenance by Phosphorylating β-CateninY654.Cell Mol Gastroenterol Hepatol. 2019;8(4):561-578. doi: 10.1016/j.jcmgh.2019.07.004. Epub 2019 Jul 19. Cell Mol Gastroenterol Hepatol. 2019. PMID: 31330317 Free PMC article.
-
Acinar-to-Ductal Metaplasia (ADM): On the Road to Pancreatic Intraepithelial Neoplasia (PanIN) and Pancreatic Cancer.Int J Mol Sci. 2023 Jun 9;24(12):9946. doi: 10.3390/ijms24129946. Int J Mol Sci. 2023. PMID: 37373094 Free PMC article. Review.
-
[New insights into the origin of pancreatic cancer. Role of atypical flat lesions in pancreatic carcinogenesis].Pathologe. 2012 Nov;33 Suppl 2:189-93. doi: 10.1007/s00292-012-1673-x. Pathologe. 2012. PMID: 23011021 Review. German.
Cited by
-
Cytokine CCL9 Mediates Oncogenic KRAS-Induced Pancreatic Acinar-to-Ductal Metaplasia by Promoting Reactive Oxygen Species and Metalloproteinases.Int J Mol Sci. 2024 Apr 26;25(9):4726. doi: 10.3390/ijms25094726. Int J Mol Sci. 2024. PMID: 38731942 Free PMC article.
-
Generation of Hydrogen Peroxide and Downstream Protein Kinase D1 Signaling Is a Common Feature of Inducers of Pancreatic Acinar-to-Ductal Metaplasia.Antioxidants (Basel). 2022 Jan 8;11(1):137. doi: 10.3390/antiox11010137. Antioxidants (Basel). 2022. PMID: 35052641 Free PMC article.
-
Inactivation of Notch4 Attenuated Pancreatic Tumorigenesis in Mice.Cancer Res Commun. 2022 Dec 12;2(12):1601-1616. doi: 10.1158/2767-9764.CRC-22-0106. eCollection 2022 Dec. Cancer Res Commun. 2022. PMID: 36970723 Free PMC article.
-
The roles of FOXM1 in pancreatic stem cells and carcinogenesis.Mol Cancer. 2013 Dec 10;12:159. doi: 10.1186/1476-4598-12-159. Mol Cancer. 2013. PMID: 24325450 Free PMC article. Review.
-
Lunatic Fringe is a potent tumor suppressor in Kras-initiated pancreatic cancer.Oncogene. 2016 May 12;35(19):2485-95. doi: 10.1038/onc.2015.306. Epub 2015 Aug 17. Oncogene. 2016. PMID: 26279302
References
-
- Bardeesy N, DePinho RA (2002) Pancreatic cancer biology and genetics. Nat Rev Cancer 2: 897–909. - PubMed
-
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, et al. (2003) Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4: 437–450. - PubMed
-
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, et al. (2007) Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11: 291–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

