Muramyl dipeptide responsive pathways in Crohn's disease: from NOD2 and beyond
- PMID: 23275943
- PMCID: PMC11113952
- DOI: 10.1007/s00018-012-1246-4
Muramyl dipeptide responsive pathways in Crohn's disease: from NOD2 and beyond
Abstract
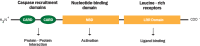
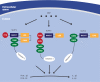
Crohn's disease (CD) is one of main disease entities under the umbrella term chronic inflammatory bowel disease. The etiology of CD involves alterations in genetic, microbiological, and immunological factors. This review is devoted to the role of the bacterial wall compound muramyl dipeptide (MDP) for the activation of inflammatory pathways involved in the pathogenesis of CD. The importance of this molecule is underscored by the fact that (1) MDP, which is found in most Gram-negative and -positive bacteria, is able to trigger several immunological responses in the intestinal system, and (2) that alterations in several mediators of the MDP response including-but not restricted to-nucleotide oligomerization domain 2 (NOD2) are associated with CD. The normalization of MDP signaling is one of several important factors that influence the intestinal inflammatory response, a fact which emphasizes the pathogenic importance of MDP signaling for the pathogenesis of CD. The important aspects of NOD2 and non-NOD2 mediated effects of MDP for the development of CD are highlighted, as well as how alterations in these pathways might translate into the development of new therapeutic strategies.
Figures


Similar articles
-
Species-specific engagement of human nucleotide oligomerization domain 2 (NOD)2 and Toll-like receptor (TLR) signalling upon intracellular bacterial infection: role of Crohn's associated NOD2 gene variants.Clin Exp Immunol. 2015 Mar;179(3):426-34. doi: 10.1111/cei.12471. Clin Exp Immunol. 2015. PMID: 25335775 Free PMC article.
-
Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection.J Biol Chem. 2003 Mar 14;278(11):8869-72. doi: 10.1074/jbc.C200651200. Epub 2003 Jan 13. J Biol Chem. 2003. PMID: 12527755
-
MDP-Induced selective tolerance to TLR4 ligands: impairment in NOD2 mutant Crohn's disease patients.Inflamm Bowel Dis. 2009 Nov;15(11):1686-96. doi: 10.1002/ibd.21013. Inflamm Bowel Dis. 2009. PMID: 19572373
-
Freund's adjuvant, NOD2 and mycobacteria.Curr Opin Microbiol. 2015 Feb;23:126-32. doi: 10.1016/j.mib.2014.11.015. Epub 2014 Dec 5. Curr Opin Microbiol. 2015. PMID: 25483349 Review.
-
Structure-activity relationship in NOD2 agonistic muramyl dipeptides.Eur J Med Chem. 2024 May 5;271:116439. doi: 10.1016/j.ejmech.2024.116439. Epub 2024 Apr 20. Eur J Med Chem. 2024. PMID: 38691886 Review.
Cited by
-
Ex vivo immunosuppressive effects of mesenchymal stem cells on Crohn's disease mucosal T cells are largely dependent on indoleamine 2,3-dioxygenase activity and cell-cell contact.Stem Cell Res Ther. 2015 Jul 24;6(1):137. doi: 10.1186/s13287-015-0122-1. Stem Cell Res Ther. 2015. PMID: 26206376 Free PMC article.
-
Pathogenesis of Crohn's disease.F1000Prime Rep. 2015 Apr 2;7:44. doi: 10.12703/P7-44. eCollection 2015. F1000Prime Rep. 2015. PMID: 26097717 Free PMC article. Review.
-
The Role of the Bacterial Muramyl Dipeptide in the Regulation of GLP-1 and Glycemia.Int J Mol Sci. 2020 Jul 24;21(15):5252. doi: 10.3390/ijms21155252. Int J Mol Sci. 2020. PMID: 32722085 Free PMC article.
-
ATG16L1: A multifunctional susceptibility factor in Crohn disease.Autophagy. 2015 Apr 3;11(4):585-94. doi: 10.1080/15548627.2015.1017187. Autophagy. 2015. PMID: 25906181 Free PMC article. Review.
-
Autophagy and checkpoints for intracellular pathogen defense.Curr Opin Gastroenterol. 2015 Jan;31(1):14-23. doi: 10.1097/MOG.0000000000000134. Curr Opin Gastroenterol. 2015. PMID: 25394238 Free PMC article. Review.
References
-
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. - PubMed
-
- Holler E, Rogler G, Herfarth H, Brenmoehl J, Wild PJ, Hahn J, Eissner G, Scholmerich J, et al. Both donor and recipient NOD2/CARD15 mutations associate with transplant-related mortality and GvHD following allogeneic stem cell transplantation. Blood. 2004;104:889–894. - PubMed
-
- Brenmoehl J, Herfarth H, Gluck T, Audebert F, Barlage S, Schmitz G, Froehlich D, Schreiber S, et al. Genetic variants in the NOD2/CARD15 gene are associated with early mortality in sepsis patients. Intensive Care Med. 2007;33:1541–1548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

