N-cadherin expression is regulated by UTP in schwannoma cells
- PMID: 23271561
- PMCID: PMC3646127
- DOI: 10.1007/s11302-012-9348-x
N-cadherin expression is regulated by UTP in schwannoma cells
Abstract
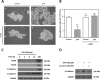
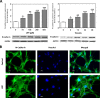
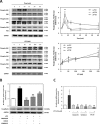
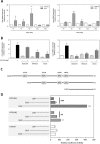
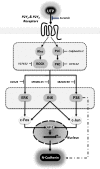
Schwann cells (SCs) are peripheral myelinating glial cells that express the neuronal Ca(2+)-dependent cell adhesion molecule, neural cadherin (N-cadherin). N-cadherin is involved in glia-glia and axon-glia interactions and participates in many key events, which range from the control of axonal growth and guidance to synapse formation and plasticity. Extracellular UTP activates P2Y purinergic receptors and exerts short- and long-term effects on several tissues to promote wound healing. Nevertheless, the contribution of P2Y receptors in peripheral nervous system functions is not completely understood. The current study demonstrated that UTP induced a dose- and time-dependent increase in N-cadherin expression in SCs. Furthermore, N-cadherin expression was blocked by the P2 purinoceptor antagonist suramin. The increased N-cadherin expression induced by UTP was mediated by phosphorylation of mitogen-activated protein kinases (MAPKs), such as Jun N-terminal kinase, extracellular-regulated kinase and p38 kinase. Moreover, the Rho kinase inhibitor Y27632, the phospholipase C inhibitor U73122 and the protein kinase C inhibitor calphostin C attenuated the UTP-induced activation of MAPKs significantly. Extracellular UTP also modulated increased in the expression of the early transcription factors c-Fos and c-Jun. We also demonstrated that the region of the N-cadherin promoter between nucleotide positions -3698 and -2620, which contained one activator protein-1-binding site, was necessary for UTP-induced gene expression. These results suggest a novel role for P2Y purinergic receptors in the regulation of N-cadherin expression in SCs.
Figures

Similar articles
-
Purinergic signaling in peripheral nervous system glial cells.Glia. 2021 Aug;69(8):1837-1851. doi: 10.1002/glia.23969. Epub 2021 Jan 28. Glia. 2021. PMID: 33507559 Free PMC article. Review.
-
Uridine 5'-triphosphate promotes in vitro Schwannoma cell migration through matrix metalloproteinase-2 activation.PLoS One. 2014 Jun 6;9(6):e98998. doi: 10.1371/journal.pone.0098998. eCollection 2014. PLoS One. 2014. PMID: 24905332 Free PMC article.
-
Secretion of ATP from Schwann cells in response to uridine triphosphate.Eur J Neurosci. 2005 Jan;21(1):151-60. doi: 10.1111/j.1460-9568.2004.03831.x. Eur J Neurosci. 2005. PMID: 15654852
-
Uridine-5'-Triphosphate Partially Blocks Differentiation Signals and Favors a more Repair State in Cultured rat Schwann Cells.Neuroscience. 2018 Feb 21;372:255-265. doi: 10.1016/j.neuroscience.2018.01.010. Epub 2018 Jan 11. Neuroscience. 2018. PMID: 29337237
-
UTP affects the Schwannoma cell line proteome through P2Y receptors leading to cytoskeletal reorganisation.Proteomics. 2012 Jan;12(1):145-56. doi: 10.1002/pmic.201100187. Epub 2011 Dec 9. Proteomics. 2012. PMID: 22065602
Cited by
-
Purinergic signaling in peripheral nervous system glial cells.Glia. 2021 Aug;69(8):1837-1851. doi: 10.1002/glia.23969. Epub 2021 Jan 28. Glia. 2021. PMID: 33507559 Free PMC article. Review.
-
Rab23 Regulates Radial Migration of Projection Neurons via N-cadherin.Cereb Cortex. 2018 Apr 1;28(4):1516-1531. doi: 10.1093/cercor/bhy018. Cereb Cortex. 2018. PMID: 29420702 Free PMC article.
-
Uridine 5'-triphosphate promotes in vitro Schwannoma cell migration through matrix metalloproteinase-2 activation.PLoS One. 2014 Jun 6;9(6):e98998. doi: 10.1371/journal.pone.0098998. eCollection 2014. PLoS One. 2014. PMID: 24905332 Free PMC article.
-
The beneficial effect of chitooligosaccharides on cell behavior and function of primary Schwann cells is accompanied by up-regulation of adhesion proteins and neurotrophins.Neurochem Res. 2014 Nov;39(11):2047-57. doi: 10.1007/s11064-014-1387-y. Epub 2014 Aug 15. Neurochem Res. 2014. PMID: 25119164
-
Molecular and nanoscale evaluation of N-cadherin expression in invasive bladder cancer cells under control conditions or GW501516 exposure.Mol Cell Biochem. 2020 Aug;471(1-2):113-127. doi: 10.1007/s11010-020-03771-1. Epub 2020 Jun 9. Mol Cell Biochem. 2020. PMID: 32519230 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

