Magnetic nanoparticle mediated enhancement of localized surface plasmon resonance for ultrasensitive bioanalytical assay in human blood plasma
- PMID: 23267460
- PMCID: PMC3565058
- DOI: 10.1021/ac302422k
Magnetic nanoparticle mediated enhancement of localized surface plasmon resonance for ultrasensitive bioanalytical assay in human blood plasma
Abstract
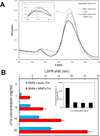
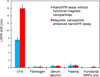
We demonstrate that Fe(3)O(4) magnetic nanoparticle (MNP) can greatly enhance the localized surface plasmon resonance (LSPR) of metal nanoparticle. The high refractive index and molecular weight of the Fe(3)O(4) MNPs make them a powerful enhancer for plasmonic response to biological binding events, thereby enabling a significant improvement in the sensitivity, reliability, dynamic range, and calibration linearity for LSPR assay of small molecules in a trace amount. Rather than using fluorescence spectroscopy or magnetic resonance imaging, this study marks the first use of the label-free LSPR nanosensor for a disease biomarker in physiological solutions, providing a low cost, clinical-oriented detection. This facile and ultrasensitive nanosensor with an extremely light, robust, and low-cost instrument is attractive for miniaturization on a lab-on-a-chip system to deliver point-of-care medical diagnostics. To further evaluate the practical application of Fe(3)O(4) MNPs in the enhancement of LSPR assay, cardiac troponin I (cTnI) for myocardial infarction diagnosis was used as a model protein to be detected by a gold nanorod (GNR) bioprobe. MNP-captured cTnI molecules resulted in spectral responses up to 6-fold higher than direct cTnI adsorption on the GNR sensor. The detection limit (LOD) was lowered to ca. 30 pM for plasma samples which is 3 orders lower than a comparable study. To the best of our knowledge, this marks the lowest LOD for a real plasma protein detection based on label-free LSPR shift without complicated instrumentation. The observed LSPR sensing enhancement by Fe(3)O(4) MNPs is independent of nonspecific binding.
Figures



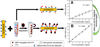
Similar articles
-
Water dispersion of magnetic nanoparticles with selective Biofunctionality for enhanced plasmonic biosensing.Talanta. 2016 May 1;151:23-29. doi: 10.1016/j.talanta.2016.01.007. Epub 2016 Jan 8. Talanta. 2016. PMID: 26946006 Free PMC article.
-
Magnetic nanoparticle enhanced surface plasmon resonance sensing and its application for the ultrasensitive detection of magnetic nanoparticle-enriched small molecules.Anal Chem. 2010 Aug 15;82(16):6782-9. doi: 10.1021/ac100812c. Anal Chem. 2010. PMID: 20704367
-
Quantification of cardiac biomarkers using label-free and multiplexed gold nanorod bioprobes for myocardial infarction diagnosis.Biosens Bioelectron. 2014 Nov 15;61:70-5. doi: 10.1016/j.bios.2014.04.043. Epub 2014 May 14. Biosens Bioelectron. 2014. PMID: 24858675 Free PMC article.
-
Plasmonic Nanoparticle-Enhanced Optical Techniques for Cancer Biomarker Sensing.Biosensors (Basel). 2023 Nov 8;13(11):977. doi: 10.3390/bios13110977. Biosensors (Basel). 2023. PMID: 37998152 Free PMC article. Review.
-
Localized surface plasmon resonance: a unique property of plasmonic nanoparticles for nucleic acid detection.Nanoscale. 2013 Dec 21;5(24):12043-71. doi: 10.1039/c3nr02257a. Nanoscale. 2013. PMID: 24166199 Review.
Cited by
-
Recent progress in nanomaterial-based electrochemical biosensors for pathogenic bacteria.Mikrochim Acta. 2019 Nov 21;186(12):820. doi: 10.1007/s00604-019-3966-8. Mikrochim Acta. 2019. PMID: 31748898 Review.
-
Gold Nanorods for LSPR Biosensing: Synthesis, Coating by Silica, and Bioanalytical Applications.Biosensors (Basel). 2020 Oct 17;10(10):146. doi: 10.3390/bios10100146. Biosensors (Basel). 2020. PMID: 33080925 Free PMC article. Review.
-
A new bifunctional hybrid nanostructure as an active platform for photothermal therapy and MR imaging.Sci Rep. 2016 Jun 14;6:27847. doi: 10.1038/srep27847. Sci Rep. 2016. PMID: 27297588 Free PMC article.
-
Cardiovascular biomarkers in body fluids: progress and prospects in optical sensors.Biophys Rev. 2022 Aug 18;14(4):1023-1050. doi: 10.1007/s12551-022-00990-2. eCollection 2022 Aug. Biophys Rev. 2022. PMID: 35996626 Free PMC article. Review.
-
Magnetic Particle Imaging-Guided Hyperthermia for Precise Treatment of Cancer: Review, Challenges, and Prospects.Mol Imaging Biol. 2023 Dec;25(6):1020-1033. doi: 10.1007/s11307-023-01856-z. Epub 2023 Oct 3. Mol Imaging Biol. 2023. PMID: 37789103 Review.
References
-
- Mie G. Ann.Phys. 1908;25:377–445.
-
- Willets KA, Van Duyne RP. Annu.Rev.Phys.Chem. 2007;58:267–297. - PubMed
-
- Lee KS, El Sayed MA. J.Phys.Chem.B. 2006;110:19220–19225. - PubMed
-
- Jain PK, Lee KS, El Sayed IH, El Sayed MA. J.Phys.Chem.B. 2006;110:7238–7248. - PubMed
-
- Link S, El Sayed MA. Annu.Rev.Phys.Chem. 2003;54:331–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

