Computational quest for understanding the role of astrocyte signaling in synaptic transmission and plasticity
- PMID: 23267326
- PMCID: PMC3528083
- DOI: 10.3389/fncom.2012.00098
Computational quest for understanding the role of astrocyte signaling in synaptic transmission and plasticity
Abstract
The complexity of the signaling network that underlies astrocyte-synapse interactions may seem discouraging when tackled from a theoretical perspective. Computational modeling is challenged by the fact that many details remain hitherto unknown and conventional approaches to describe synaptic function are unsuitable to explain experimental observations when astrocytic signaling is taken into account. Supported by experimental evidence is the possibility that astrocytes perform genuine information processing by means of their calcium signaling and are players in the physiological setting of the basal tone of synaptic transmission. Here we consider the plausibility of this scenario from a theoretical perspective, focusing on the modulation of synaptic release probability by the astrocyte and its implications on synaptic plasticity. The analysis of the signaling pathways underlying such modulation refines our notion of tripartite synapse and has profound implications on our understanding of brain function.
Keywords: astrocyte modeling; astrocyte-synapse interactions; calcium encoding; calcium signaling; cortical maps; gliotransmission; metaplasticity; synaptic plasticity.
Figures
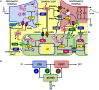

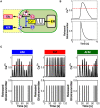

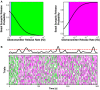
Similar articles
-
Diversity and Specificity of Astrocyte-neuron Communication.Neuroscience. 2019 Jan 1;396:73-78. doi: 10.1016/j.neuroscience.2018.11.010. Epub 2018 Nov 17. Neuroscience. 2019. PMID: 30458223 Free PMC article. Review.
-
Lateral regulation of synaptic transmission by astrocytes.Neuroscience. 2016 May 26;323:62-6. doi: 10.1016/j.neuroscience.2015.02.036. Epub 2015 Feb 27. Neuroscience. 2016. PMID: 25732135
-
A Computational Model of Interactions Between Neuronal and Astrocytic Networks: The Role of Astrocytes in the Stability of the Neuronal Firing Rate.Front Comput Neurosci. 2020 Jan 22;13:92. doi: 10.3389/fncom.2019.00092. eCollection 2019. Front Comput Neurosci. 2020. PMID: 32038210 Free PMC article.
-
Modeling synaptic transmission of the tripartite synapse.Phys Biol. 2007 Jan 9;4(1):1-9. doi: 10.1088/1478-3975/4/1/001. Phys Biol. 2007. PMID: 17406080
-
Astrocyte plasticity: implications for synaptic and neuronal activity.Neuroscientist. 2013 Dec;19(6):604-15. doi: 10.1177/1073858413504999. Epub 2013 Oct 10. Neuroscientist. 2013. PMID: 24122819 Review.
Cited by
-
Mechanisms of heterosynaptic metaplasticity.Philos Trans R Soc Lond B Biol Sci. 2013 Dec 2;369(1633):20130148. doi: 10.1098/rstb.2013.0148. Print 2014 Jan 5. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 24298150 Free PMC article. Review.
-
Astrocyte-mediated neuronal irregularities and dynamics: the complexity of the tripartite synapse.Biol Cybern. 2024 Dec;118(5-6):249-266. doi: 10.1007/s00422-024-00994-z. Epub 2024 Sep 14. Biol Cybern. 2024. PMID: 39276225
-
Astrocytic Modulation of Neuronal Activity in the Suprachiasmatic Nucleus: Insights from Mathematical Modeling.J Biol Rhythms. 2020 Jun;35(3):287-301. doi: 10.1177/0748730420913672. Epub 2020 Apr 14. J Biol Rhythms. 2020. PMID: 32285754 Free PMC article.
-
The brain as a "hyper-network": the key role of neural networks as main producers of the integrated brain actions especially via the "broadcasted" neuroconnectomics.J Neural Transm (Vienna). 2018 Jun;125(6):883-897. doi: 10.1007/s00702-018-1855-7. Epub 2018 Feb 9. J Neural Transm (Vienna). 2018. PMID: 29427068 Review.
-
Glutamate mediated astrocytic filtering of neuronal activity.PLoS Comput Biol. 2014 Dec 18;10(12):e1003964. doi: 10.1371/journal.pcbi.1003964. eCollection 2014 Dec. PLoS Comput Biol. 2014. PMID: 25521344 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

