Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 promotes transcription
- PMID: 23267096
- PMCID: PMC3545798
- DOI: 10.1073/pnas.1208446110
Deubiquitination of NF-κB by Ubiquitin-Specific Protease-7 promotes transcription
Abstract
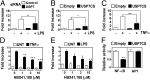
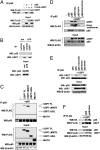
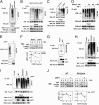
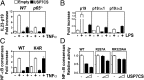
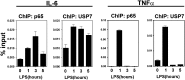
NF-κB is the master regulator of the immune response and is responsible for the transcription of hundreds of genes controlling inflammation and immunity. Activation of NF-κB occurs in the cytoplasm through the kinase activity of the IκB kinase complex, which leads to translocation of NF-κB to the nucleus. Once in the nucleus, NF-κB transcriptional activity is regulated by DNA binding-dependent ubiquitin-mediated proteasomal degradation. We have identified the deubiquitinase Ubiquitin Specific Protease-7 (USP7) as a regulator of NF-κB transcriptional activity. USP7 deubiquitination of NF-κB leads to increased transcription. Loss of USP7 activity results in increased ubiquitination of NF-κB, leading to reduced promoter occupancy and reduced expression of target genes in response to Toll-like- and TNF-receptor activation. These findings reveal a unique mechanism controlling NF-κB activity and demonstrate that the deubiquitination of NF-κB by USP7 is critical for target gene transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
TRAF Family Member-associated NF-κB Activator (TANK) Inhibits Genotoxic Nuclear Factor κB Activation by Facilitating Deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase.J Biol Chem. 2015 May 22;290(21):13372-85. doi: 10.1074/jbc.M115.643767. Epub 2015 Apr 10. J Biol Chem. 2015. PMID: 25861989 Free PMC article.
-
HSCARG downregulates NF-κB signaling by interacting with USP7 and inhibiting NEMO ubiquitination.Cell Death Dis. 2014 May 15;5(5):e1229. doi: 10.1038/cddis.2014.197. Cell Death Dis. 2014. PMID: 24832601 Free PMC article.
-
The deubiquitinase USP7 uses a distinct ubiquitin-like domain to deubiquitinate NF-ĸB subunits.J Biol Chem. 2020 Aug 14;295(33):11754-11763. doi: 10.1074/jbc.RA120.014113. Epub 2020 Jun 25. J Biol Chem. 2020. PMID: 32587091 Free PMC article.
-
Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
Cited by
-
The Deubiquitinating Enzyme USP7 Regulates Androgen Receptor Activity by Modulating Its Binding to Chromatin.J Biol Chem. 2015 Aug 28;290(35):21713-23. doi: 10.1074/jbc.M114.628255. Epub 2015 Jul 14. J Biol Chem. 2015. PMID: 26175158 Free PMC article.
-
Regulation of NFκB Signalling by Ubiquitination: A Potential Therapeutic Target in Head and Neck Squamous Cell Carcinoma?Cancers (Basel). 2020 Oct 7;12(10):2877. doi: 10.3390/cancers12102877. Cancers (Basel). 2020. PMID: 33036368 Free PMC article. Review.
-
Long noncoding RNA LINC02582 acts downstream of miR-200c to promote radioresistance through CHK1 in breast cancer cells.Cell Death Dis. 2019 Oct 10;10(10):764. doi: 10.1038/s41419-019-1996-0. Cell Death Dis. 2019. PMID: 31601781 Free PMC article.
-
USP7 is a SUMO deubiquitinase essential for DNA replication.Nat Struct Mol Biol. 2016 Apr;23(4):270-7. doi: 10.1038/nsmb.3185. Epub 2016 Mar 7. Nat Struct Mol Biol. 2016. PMID: 26950370 Free PMC article.
-
Deubiquitinating Enzymes Orchestrate the Cancer Stem Cell-Immunosuppressive Niche Dialogue: New Perspectives and Therapeutic Potential.Front Cell Dev Biol. 2021 Jun 9;9:680100. doi: 10.3389/fcell.2021.680100. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34179009 Free PMC article. Review.
References
-
- Carmody RJ, Chen YH. Nuclear factor-kappaB: Activation and regulation during toll-like receptor signaling. Cell Mol Immunol. 2007;4(1):31–41. - PubMed
-
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–362. - PubMed
-
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8(11):837–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

