The laminin family
- PMID: 23263632
- PMCID: PMC3544786
- DOI: 10.4161/cam.22826
The laminin family
Abstract
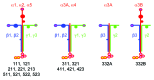
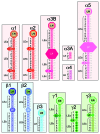
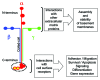
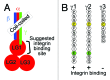
Laminins are large molecular weight glycoproteins constituted by the assembly of three disulfide-linked polypeptides, the α, β and γ chains. The human genome encodes 11 genetically distinct laminin chains. Structurally, laminin chains differ by the number, size and organization of a few constitutive domains, endowing the various members of the laminin family with common and unique important functions. In particular, laminins are indispensable building blocks for cellular networks physically bridging the intracellular and extracellular compartments and relaying signals critical for cellular behavior, and for extracellular polymers determining the architecture and the physiology of basement membranes.
Figures
Similar articles
-
The laminins.Matrix Biol. 1994 Aug;14(4):275-81. doi: 10.1016/0945-053x(94)90192-9. Matrix Biol. 1994. PMID: 7827749 Review.
-
Laminins in basement membrane assembly.Cell Adh Migr. 2013 Jan-Feb;7(1):56-63. doi: 10.4161/cam.21831. Epub 2012 Oct 17. Cell Adh Migr. 2013. PMID: 23076216 Free PMC article. Review.
-
Vascular laminins in physiology and pathology.Matrix Biol. 2017 Jan;57-58:140-148. doi: 10.1016/j.matbio.2016.06.008. Epub 2016 Jul 2. Matrix Biol. 2017. PMID: 27378388 Review. No abstract available.
-
Molecular analysis of laminin N-terminal domains mediating self-interactions.J Biol Chem. 2004 Oct 22;279(43):44504-12. doi: 10.1074/jbc.M402455200. Epub 2004 Aug 13. J Biol Chem. 2004. PMID: 15310759
-
Laminin 332 processing impacts cellular behavior.Cell Adh Migr. 2013 Jan-Feb;7(1):122-34. doi: 10.4161/cam.23132. Epub 2012 Dec 21. Cell Adh Migr. 2013. PMID: 23263634 Free PMC article. Review.
Cited by
-
LAMC2 as a prognostic biomarker in human cancer: a systematic review and meta-analysis.BMJ Open. 2022 Nov 17;12(11):e063682. doi: 10.1136/bmjopen-2022-063682. BMJ Open. 2022. PMID: 36396303 Free PMC article.
-
Bioactive extracellular matrix fragments in lung health and disease.J Clin Invest. 2016 Sep 1;126(9):3176-84. doi: 10.1172/JCI83147. Epub 2016 Sep 1. J Clin Invest. 2016. PMID: 27584731 Free PMC article. Review.
-
ADAMTS18 deficiency associates extracellular matrix dysfunction with a higher risk of HER2-positive mammary tumorigenesis and metastasis.Breast Cancer Res. 2024 Jan 29;26(1):19. doi: 10.1186/s13058-024-01771-3. Breast Cancer Res. 2024. PMID: 38287441 Free PMC article.
-
Laminin: loss-of-function studies.Cell Mol Life Sci. 2017 Mar;74(6):1095-1115. doi: 10.1007/s00018-016-2381-0. Epub 2016 Oct 1. Cell Mol Life Sci. 2017. PMID: 27696112 Free PMC article. Review.
-
Colorectal cancer and basement membranes: clinicopathological correlations.Gastroenterol Res Pract. 2014;2014:580159. doi: 10.1155/2014/580159. Epub 2014 Dec 28. Gastroenterol Res Pract. 2014. PMID: 25614736 Free PMC article. Review.
References
-
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
