Decreased parkin solubility is associated with impairment of autophagy in the nigrostriatum of sporadic Parkinson's disease
- PMID: 23262240
- PMCID: PMC3618990
- DOI: 10.1016/j.neuroscience.2012.12.018
Decreased parkin solubility is associated with impairment of autophagy in the nigrostriatum of sporadic Parkinson's disease
Abstract
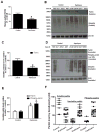
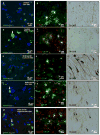
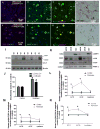
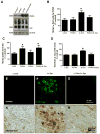
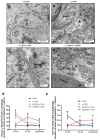
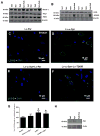
Parkinson's disease (PD) is a motor disorder that involves death of dopaminergic neurons in the substantia nigra pars compacta. Parkin is an autosomal recessive gene that is mutated in early onset PD. We investigated the role of parkin and autophagic clearance in postmortem nigrostriatal tissues from 22 non-familial sporadic PD patients and 15 control samples. Parkin was insoluble with altered cytosolic expression in the nigrostriatum of sporadic PD. Parkin insolubility was associated with lack of degradation of ubiquitinated proteins and accumulation of α-Synuclein and parkin in autophagosomes, suggesting autophagic defects in PD. To test parkin's role in mediating autophagic clearance, we used lentiviral gene transfer to express human wild type or mutant parkin (T240R) with α-Synuclein in the rat striatum. Lentiviral expression of α-Synuclein led to accumulation of autophagic vacuoles, while co-expression of parkin with α-Synuclein facilitated autophagic clearance. Subcellular fractionation showed accumulation of α-Synuclein and tau hyper-phosphorylation (p-Tau) in autophagosomes in gene transfer models, similar to the effects observed in PD brains, but parkin expression led to protein deposition into lysosomes. However, parkin loss of function mutation did not affect autophagic clearance. Taken together, these data suggest that functional parkin regulates autophagosome clearance, while decreased parkin solubility may alter normal autophagy in sporadic PD.
Copyright © 2012 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Ubiquitination increases parkin activity to promote autophagic α-synuclein clearance.PLoS One. 2013 Dec 26;8(12):e83914. doi: 10.1371/journal.pone.0083914. eCollection 2013. PLoS One. 2013. PMID: 24386307 Free PMC article.
-
Diminished parkin solubility and co-localization with intraneuronal amyloid-β are associated with autophagic defects in Alzheimer's disease.J Alzheimers Dis. 2013;33(1):231-47. doi: 10.3233/JAD-2012-121141. J Alzheimers Dis. 2013. PMID: 22954671
-
Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease.Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17510-5. doi: 10.1073/pnas.0405313101. Epub 2004 Dec 2. Proc Natl Acad Sci U S A. 2004. PMID: 15576511 Free PMC article.
-
The interplay between parkin and alpha-synuclein; possible implications for the pathogenesis of Parkinson's disease.Acta Neurobiol Exp (Wars). 2019;79(3):276-289. Acta Neurobiol Exp (Wars). 2019. PMID: 31587020 Review.
-
The regulatory role of α-synuclein and parkin in neuronal cell apoptosis; possible implications for the pathogenesis of Parkinson's disease.Apoptosis. 2010 Nov;15(11):1312-21. doi: 10.1007/s10495-010-0486-8. Apoptosis. 2010. PMID: 20221696 Review.
Cited by
-
Biochemical study of the effect of mesenchymal stem cells-derived exosome versus L-Dopa in experimentally induced Parkinson's disease in rats.Mol Cell Biochem. 2023 Dec;478(12):2795-2811. doi: 10.1007/s11010-023-04700-8. Epub 2023 Mar 26. Mol Cell Biochem. 2023. PMID: 36966421 Free PMC article.
-
Ubiquitination increases parkin activity to promote autophagic α-synuclein clearance.PLoS One. 2013 Dec 26;8(12):e83914. doi: 10.1371/journal.pone.0083914. eCollection 2013. PLoS One. 2013. PMID: 24386307 Free PMC article.
-
Activating Autophagy as a Therapeutic Strategy for Parkinson's Disease.CNS Drugs. 2018 Jan;32(1):1-11. doi: 10.1007/s40263-018-0497-5. CNS Drugs. 2018. PMID: 29492779 Review.
-
Two sides of the same coin: tyrosine kinase inhibition in cancer and neurodegeneration.Neural Regen Res. 2015 Nov;10(11):1767-9. doi: 10.4103/1673-5374.165320. Neural Regen Res. 2015. PMID: 26807110 Free PMC article. No abstract available.
-
Tyrosine Kinase Inhibition Regulates Early Systemic Immune Changes and Modulates the Neuroimmune Response in α-Synucleinopathy.J Clin Cell Immunol. 2014 Sep 30;5:259. doi: 10.4172/2155-9899.1000259. J Clin Cell Immunol. 2014. PMID: 25635231 Free PMC article.
References
-
- Avraham E, Rott R, Liani E, Szargel R, Engelender S. Phosphorylation of Parkin by the cyclin-dependent kinase 5 at the linker region modulates its ubiquitin-ligase activity and aggregation. J Biol Chem. 2007;282:12842–12850. - PubMed
-
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

