The myosin motor Myo1c is required for VEGFR2 delivery to the cell surface and for angiogenic signaling
- PMID: 23262137
- PMCID: PMC3602755
- DOI: 10.1152/ajpheart.00744.2012
The myosin motor Myo1c is required for VEGFR2 delivery to the cell surface and for angiogenic signaling
Abstract
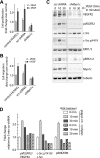
Vascular endothelial growth factor receptor-2 (VEGFR2) is a receptor tyrosine kinase that is expressed in endothelial cells and regulates angiogenic signal transduction under both physiological and pathological conditions. VEGFR2 turnover at the plasma membrane (PM) is regulated by its transport through endocytic and secretory transport pathways. Short-range cargo trafficking along actin filaments is commonly regulated by motor proteins of myosin superfamily. In the current study, performed in primary human endothelial cells, we demonstrate that unconventional myosin 1c (Myo1c; class I family member) regulates the localization of VEGFR2 at the PM. We further demonstrate that the recruitment of VEGFR2 to the PM and its colocalization with Myo1c and caveolin-1 occur in response to VEGF-A (VEGF) stimulation. In addition, VEGF-induced delivery of VEGFR2 to the cell surface requires Myo1c; surface VEGFR2 levels are reduced in the absence of Myo1c and, more importantly, are restored by the overexpression of wild-type but not mutant Myo1c. Subcellular density gradient fractionation revealed that partitioning of VEGFR2 into caveolin-1- and Myo1c-enriched membrane fractions is dependent on VEGF stimulation. Myo1c depletion resulted in increased VEGF-induced VEGFR2 transport to the lysosomes for degradation and was rescued by applying either brefeldin A, which blocks trafficking between the endoplasmic reticulum and the Golgi complex, or dynasore, an inhibitor of dynamin-mediated endocytosis. Myo1c depletion also reduced VEGF-induced VEGFR2 phosphorylation at Y1175 and phosphorylation-dependent activation of ERK1/2 and c-Src kinase, leading to reduced cell proliferation and cell migration. This is the first report demonstrating that Myo1c is an important mediator of VEGF-induced VEGFR2 delivery to the cell surface and plays a role in angiogenic signaling.
Figures

Similar articles
-
Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis.Circ Res. 2005 Mar 4;96(4):467-75. doi: 10.1161/01.RES.0000158286.51045.16. Epub 2005 Feb 3. Circ Res. 2005. PMID: 15692085
-
Loss of functional MYO1C/myosin 1c, a motor protein involved in lipid raft trafficking, disrupts autophagosome-lysosome fusion.Autophagy. 2014;10(12):2310-23. doi: 10.4161/15548627.2014.984272. Autophagy. 2014. PMID: 25551774 Free PMC article.
-
FGD5 Regulates VEGF Receptor-2 Coupling to PI3 Kinase and Receptor Recycling.Arterioscler Thromb Vasc Biol. 2017 Dec;37(12):2301-2310. doi: 10.1161/ATVBAHA.117.309978. Epub 2017 Oct 19. Arterioscler Thromb Vasc Biol. 2017. PMID: 29051140
-
VEGF-A-stimulated signalling in endothelial cells via a dual receptor tyrosine kinase system is dependent on co-ordinated trafficking and proteolysis.Biochem Soc Trans. 2009 Dec;37(Pt 6):1193-7. doi: 10.1042/BST0371193. Biochem Soc Trans. 2009. PMID: 19909245 Review.
-
Protein kinase D1, a new molecular player in VEGF signaling and angiogenesis.Mol Cells. 2009 Jul 31;28(1):1-5. doi: 10.1007/s10059-009-0109-9. Epub 2009 Jul 20. Mol Cells. 2009. PMID: 19655095 Free PMC article. Review.
Cited by
-
Landscape of heart proteome changes in a diet-induced obesity model.Sci Rep. 2019 Dec 2;9(1):18050. doi: 10.1038/s41598-019-54522-2. Sci Rep. 2019. PMID: 31792287 Free PMC article.
-
MYO1D binds with kinase domain of the EGFR family to anchor them to plasma membrane before their activation and contributes carcinogenesis.Oncogene. 2019 Dec;38(49):7416-7432. doi: 10.1038/s41388-019-0954-8. Epub 2019 Aug 16. Oncogene. 2019. PMID: 31420606
-
Convergence of VEGF and YAP/TAZ signaling: Implications for angiogenesis and cancer biology.Sci Signal. 2018 Oct 16;11(552):eaau1165. doi: 10.1126/scisignal.aau1165. Sci Signal. 2018. PMID: 30327408 Free PMC article. Review.
-
TFEB controls vascular development by regulating the proliferation of endothelial cells.EMBO J. 2019 Feb 1;38(3):e98250. doi: 10.15252/embj.201798250. Epub 2018 Dec 27. EMBO J. 2019. PMID: 30591554 Free PMC article.
-
Role of Hippo Pathway-YAP/TAZ Signaling in Angiogenesis.Front Cell Dev Biol. 2019 Apr 10;7:49. doi: 10.3389/fcell.2019.00049. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31024911 Free PMC article. Review.
References
-
- Bahler M, Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett 513: 107–113, 2002 - PubMed
-
- Ballmer-Hofer K, Andersson AE, Ratcliffe LE, Berger P. Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood 118: 816–826, 2011 - PubMed
-
- Barletta GM, Kovari IA, Verma RK, Kerjaschki D, Holzman LB. Nephrin and Neph1 co-localize at the podocyte foot process intercellular junction and form cis hetero-oligomers. J Biol Chem 278: 19266–19271, 2003 - PubMed
-
- Barylko B, Jung G, Albanesi JP. Structure, function, and regulation of myosin 1C. Acta Biochim Pol 52: 373–380, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

