Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs
- PMID: 23257360
- PMCID: PMC3533292
- DOI: 10.1172/JCI64617
Treg-mediated suppression of atherosclerosis requires MYD88 signaling in DCs
Abstract
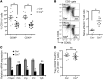
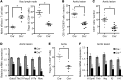
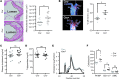
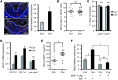
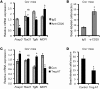
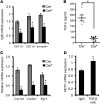
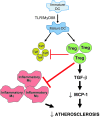
TLR activation on CD11c+ DCs triggers DC maturation, which is critical for T cell activation. Given the expansion of CD11c+ DCs during the progression of atherosclerosis and the key role of T cell activation in atherogenesis, we sought to understand the role of TLR signaling in CD11c+ DCs in atherosclerosis. To this end, we used a mouse model in which a key TLR adaptor involved in DC maturation, MYD88, is deleted in CD11c+ DCs. We transplanted bone marrow containing Myd88-deficient CD11c+ DCs into Western diet-fed LDL receptor knockout mice and found that the transplanted mice had decreased activation of effector T cells in the periphery as well as decreased infiltration of both effector T cells and Tregs in atherosclerotic lesions. Surprisingly, the net effect was an increase in atherosclerotic lesion size due to an increase in the content of myeloid-derived inflammatory cells. The mechanism involves increased lesional monocyte recruitment associated with loss of Treg-mediated suppression of MCP-1. Thus, the dominant effect of MYD88 signaling in CD11c+ DCs in the setting of atherosclerosis is to promote the development of atheroprotective Tregs. In the absence of MYD88 signaling in CD11c+ DCs, the loss of this protective Treg response trumps the loss of proatherogenic T effector cell activation.
Figures
Similar articles
-
Impaired Autophagy in CD11b+ Dendritic Cells Expands CD4+ Regulatory T Cells and Limits Atherosclerosis in Mice.Circ Res. 2019 Nov 8;125(11):1019-1034. doi: 10.1161/CIRCRESAHA.119.315248. Epub 2019 Oct 15. Circ Res. 2019. PMID: 31610723 Free PMC article.
-
Oxidized low-density lipoprotein-induced apoptotic dendritic cells as a novel therapy for atherosclerosis.J Immunol. 2015 Mar 1;194(5):2208-18. doi: 10.4049/jimmunol.1401843. Epub 2015 Feb 4. J Immunol. 2015. PMID: 25653425
-
Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling.PLoS Pathog. 2010 Aug 12;6(8):e1001045. doi: 10.1371/journal.ppat.1001045. PLoS Pathog. 2010. PMID: 20714353 Free PMC article.
-
Dendritic cell specific targeting of MyD88 signalling pathways in vivo.Eur J Immunol. 2015 Jan;45(1):32-9. doi: 10.1002/eji.201444747. Epub 2014 Dec 16. Eur J Immunol. 2015. PMID: 25403892 Review.
-
Toll-like receptors in atherosclerosis.Int J Mol Sci. 2013 Jul 4;14(7):14008-23. doi: 10.3390/ijms140714008. Int J Mol Sci. 2013. PMID: 23880853 Free PMC article. Review.
Cited by
-
TAK1 regulates Paneth cell integrity partly through blocking necroptosis.Cell Death Dis. 2016 Apr 14;7(4):e2196. doi: 10.1038/cddis.2016.98. Cell Death Dis. 2016. PMID: 27077812 Free PMC article.
-
Recent Advances on the Role and Therapeutic Potential of Regulatory T Cells in Atherosclerosis.J Clin Med. 2021 Dec 16;10(24):5907. doi: 10.3390/jcm10245907. J Clin Med. 2021. PMID: 34945203 Free PMC article. Review.
-
Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity.Circulation. 2014 Apr 22;129(16):1677-87. doi: 10.1161/CIRCULATIONAHA.113.006381. Epub 2014 Jan 31. Circulation. 2014. PMID: 24488984 Free PMC article.
-
Myeloid cells in atherosclerosis: a delicate balance of anti-inflammatory and proinflammatory mechanisms.Curr Opin Lipidol. 2013 Oct;24(5):371-80. doi: 10.1097/MOL.0b013e328363d298. Curr Opin Lipidol. 2013. PMID: 24005215 Free PMC article. Review.
-
Mechanisms that regulate macrophage burden in atherosclerosis.Circ Res. 2014 May 23;114(11):1757-71. doi: 10.1161/CIRCRESAHA.114.301174. Circ Res. 2014. PMID: 24855200 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

