Oxidative metabolites of curcumin poison human type II topoisomerases
- PMID: 23253398
- PMCID: PMC3541001
- DOI: 10.1021/bi3014455
Oxidative metabolites of curcumin poison human type II topoisomerases
Abstract
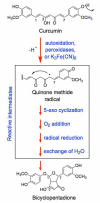
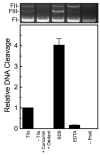
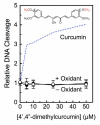
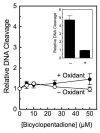
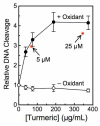
The polyphenol curcumin is the principal flavor and color component of the spice turmeric. Beyond its culinary uses, curcumin is believed to positively impact human health and displays antioxidant, anti-inflammatory, antibacterial, and chemopreventive properties. It also is in clinical trials as an anticancer agent. In aqueous solution at physiological pH, curcumin undergoes spontaneous autoxidation that is enhanced by oxidizing agents. The reaction proceeds through a series of quinone methide and other reactive intermediates to form a final dioxygenated bicyclopentadione product. Several naturally occurring polyphenols that can form quinones have been shown to act as topoisomerase II poisons (i.e., they increase levels of topoisomerase II-mediated DNA cleavage). Because several of these compounds have chemopreventive properties, we determined the effects of curcumin, its oxidative metabolites, and structurally related degradation products (vanillin, ferulic acid, and feruloylmethane) on the DNA cleavage activities of human topoisomerase IIα and IIβ. Intermediates in the curcumin oxidation pathway increased the level of DNA scission mediated by both enzymes ~4-5-fold. In contrast, curcumin and the bicyclopentadione, as well as vanillin, ferulic acid, and feruloylmethane, had no effect on DNA cleavage. As found for other quinone-based compounds, curcumin oxidation intermediates acted as redox-dependent (as opposed to interfacial) topoisomerase II poisons. Finally, under conditions that promote oxidation, the dietary spice turmeric enhanced topoisomerase II-mediated DNA cleavage. Thus, even within the more complex spice formulation, oxidized curcumin intermediates appear to function as topoisomerase II poisons.
Figures




Similar articles
-
Oxidative Transformation of Demethoxy- and Bisdemethoxycurcumin: Products, Mechanism of Formation, and Poisoning of Human Topoisomerase IIα.Chem Res Toxicol. 2015 May 18;28(5):989-96. doi: 10.1021/acs.chemrestox.5b00009. Epub 2015 Apr 3. Chem Res Toxicol. 2015. PMID: 25806475 Free PMC article.
-
Effects of Olive Metabolites on DNA Cleavage Mediated by Human Type II Topoisomerases.Biochemistry. 2015 Jul 28;54(29):4531-41. doi: 10.1021/acs.biochem.5b00162. Epub 2015 Jul 13. Biochemistry. 2015. PMID: 26132160 Free PMC article.
-
1,2-Naphthoquinone as a Poison of Human Type II Topoisomerases.Chem Res Toxicol. 2021 Apr 19;34(4):1082-1090. doi: 10.1021/acs.chemrestox.0c00492. Epub 2021 Mar 24. Chem Res Toxicol. 2021. PMID: 33760604 Free PMC article.
-
Degradation of Curcumin: From Mechanism to Biological Implications.J Agric Food Chem. 2015 Sep 9;63(35):7606-14. doi: 10.1021/acs.jafc.5b00244. Epub 2015 Apr 2. J Agric Food Chem. 2015. PMID: 25817068 Free PMC article. Review.
-
Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives.Food Funct. 2018 Feb 21;9(2):705-714. doi: 10.1039/c7fo01242j. Food Funct. 2018. PMID: 29206254 Review.
Cited by
-
Topoisomerase II Poisons: Converting Essential Enzymes into Molecular Scissors.Biochemistry. 2021 Jun 1;60(21):1630-1641. doi: 10.1021/acs.biochem.1c00240. Epub 2021 May 19. Biochemistry. 2021. PMID: 34008964 Free PMC article. Review.
-
Oxidative Transformation of Demethoxy- and Bisdemethoxycurcumin: Products, Mechanism of Formation, and Poisoning of Human Topoisomerase IIα.Chem Res Toxicol. 2015 May 18;28(5):989-96. doi: 10.1021/acs.chemrestox.5b00009. Epub 2015 Apr 3. Chem Res Toxicol. 2015. PMID: 25806475 Free PMC article.
-
Natural products as topoisomerase II poisons: effects of thymoquinone on DNA cleavage mediated by human topoisomerase IIα.Chem Res Toxicol. 2014 May 19;27(5):787-93. doi: 10.1021/tx400453v. Epub 2014 Mar 31. Chem Res Toxicol. 2014. PMID: 24650156 Free PMC article.
-
Synthesis of novel C5-curcuminoid-fatty acid conjugates and mechanistic investigation of their anticancer activity.Bioorg Med Chem Lett. 2015;25(10):2174-80. doi: 10.1016/j.bmcl.2015.03.065. Epub 2015 Mar 31. Bioorg Med Chem Lett. 2015. PMID: 25881826 Free PMC article.
-
Curcumin Oxidation Is Required for Inhibition of Helicobacter pylori Growth, Translocation and Phosphorylation of Cag A.Front Cell Infect Microbiol. 2021 Dec 24;11:765842. doi: 10.3389/fcimb.2021.765842. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 35004346 Free PMC article.
References
-
- Jaffrey M. Madhur Jaffrey’s spice kitchenfifty recipies introducing Indian spices and aromatic seeds. Clarkson Potter; New York: 1994.
-
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. - PubMed
-
- Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol. Nutr. Food Res. 2012 Epub. Aug. 13 2012. - PubMed
-
- Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986;24:651–654. - PubMed
-
- Mahady GB, Pendland SL, Yun G, Lu ZZ. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22:4179–4181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

