β-cell metabolic alterations under chronic nutrient overload in rat and human islets
- PMID: 23247575
- PMCID: PMC3605166
- DOI: 10.4161/isl.22720
β-cell metabolic alterations under chronic nutrient overload in rat and human islets
Abstract
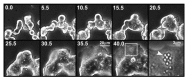
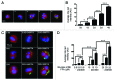
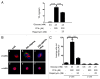
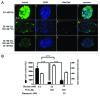
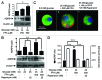
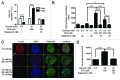
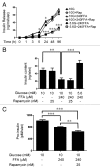
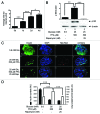
The aim of this study was to assess multifactorial β-cell responses to metabolic perturbations in primary rat and human islets. Treatment of dispersed rat islet cells with elevated glucose and free fatty acids (FFAs, oleate:palmitate = 1:1 v/v) resulted in increases in the size and the number of lipid droplets in β-cells in a time- and concentration-dependent manner. Glucose and FFAs synergistically stimulated the nutrient sensor mammalian target of rapamycin complex 1 (mTORC1). A potent mTORC1 inhibitor, rapamycin (25 nM), significantly reduced triglyceride accumulation in rat islets. Importantly, lipid droplets accumulated only in β-cells but not in α-cells in an mTORC1-dependent manner. Nutrient activation of mTORC1 upregulated the expression of adipose differentiation related protein (ADRP), known to stabilize lipid droplets. Rat islet size and new DNA synthesis also increased under nutrient overload. Insulin secretion into the culture medium increased steadily over a 4-day period without any significant difference between glucose (10 mM) alone and the combination of glucose (10 mM) and FFAs (240 μM). Insulin content and insulin biosynthesis, however, were significantly reduced under the combination of nutrients compared with glucose alone. Elevated nutrients also stimulated lipid droplet formation in human islets in an mTORC1-dependent manner. Unlike rat islets, however, human islets did not increase in size under nutrient overload despite a normal response to nutrients in releasing insulin. The different responses of islet cell growth under nutrient overload appear to impact insulin biosynthesis and storage differently in rat and human islets.
Keywords: ADRP; human islets; insulin; lipid droplets; mTORC1; nutrient overload; rapamycin; time lapse studies.
Figures

Similar articles
-
Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic beta-cells via activation of mTORC1.PLoS One. 2009;4(3):e4954. doi: 10.1371/journal.pone.0004954. Epub 2009 Mar 23. PLoS One. 2009. PMID: 19305497 Free PMC article.
-
mTORC1 inhibition via rapamycin promotes triacylglycerol lipolysis and release of free fatty acids in 3T3-L1 adipocytes.Lipids. 2010 Dec;45(12):1089-100. doi: 10.1007/s11745-010-3488-y. Epub 2010 Nov 2. Lipids. 2010. PMID: 21042876 Free PMC article.
-
Reciprocal regulation of mTOR complexes in pancreatic islets from humans with type 2 diabetes.Diabetologia. 2017 Apr;60(4):668-678. doi: 10.1007/s00125-016-4188-9. Epub 2016 Dec 21. Diabetologia. 2017. PMID: 28004151
-
mTORC2 Signaling: A Path for Pancreatic β Cell's Growth and Function.J Mol Biol. 2018 Mar 30;430(7):904-918. doi: 10.1016/j.jmb.2018.02.013. Epub 2018 Feb 23. J Mol Biol. 2018. PMID: 29481838 Review.
-
mTORC1 Signaling: A Double-Edged Sword in Diabetic β Cells.Cell Metab. 2018 Feb 6;27(2):314-331. doi: 10.1016/j.cmet.2017.11.004. Epub 2017 Dec 21. Cell Metab. 2018. PMID: 29275961 Review.
Cited by
-
Chronic Exposure to Palmitic Acid Down-Regulates AKT in Beta-Cells through Activation of mTOR.Am J Pathol. 2022 Jan;192(1):130-145. doi: 10.1016/j.ajpath.2021.09.008. Epub 2021 Oct 5. Am J Pathol. 2022. PMID: 34619135 Free PMC article.
-
Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids.Anat Cell Biol. 2015 Mar;48(1):16-24. doi: 10.5115/acb.2015.48.1.16. Epub 2015 Mar 20. Anat Cell Biol. 2015. PMID: 25806118 Free PMC article. Review.
-
CD36 mediates lipid accumulation in pancreatic beta cells under the duress of glucolipotoxic conditions: Novel roles of lysine deacetylases.Biochem Biophys Res Commun. 2018 Jan 15;495(3):2221-2226. doi: 10.1016/j.bbrc.2017.12.111. Epub 2017 Dec 20. Biochem Biophys Res Commun. 2018. PMID: 29274335 Free PMC article.
-
Adaptation of β-Cell and Endothelial Function to Carbohydrate Loading: Influence of Insulin Resistance.Diabetes. 2015 Jul;64(7):2550-9. doi: 10.2337/db15-0106. Epub 2015 Mar 9. Diabetes. 2015. PMID: 25754957 Free PMC article.
-
Perilipin 5 regulates islet lipid metabolism and insulin secretion in a cAMP-dependent manner: implication of its role in the postprandial insulin secretion.Diabetes. 2015 Apr;64(4):1299-310. doi: 10.2337/db14-0559. Epub 2014 Nov 12. Diabetes. 2015. PMID: 25392244 Free PMC article.
References
-
- Milburn JL, Jr., Hirose H, Lee YH, Nagasawa Y, Ogawa A, Ohneda M, et al. Pancreatic beta-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J Biol Chem. 1995;270:1295–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK0822574/DK/NIDDK NIH HHS/United States
- RC2 ES018781/ES/NIEHS NIH HHS/United States
- R56 DK006181/DK/NIDDK NIH HHS/United States
- R15 DK094142/DK/NIDDK NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- R01 DK006181/DK/NIDDK NIH HHS/United States
- DK06181/DK/NIDDK NIH HHS/United States
- DK20579/DK/NIDDK NIH HHS/United States
- 1R15DK094142/DK/NIDDK NIH HHS/United States
- R24 DK087669/DK/NIDDK NIH HHS/United States
- 1RC2ES018781/ES/NIEHS NIH HHS/United States
- DK00618146S1/DK/NIDDK NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
