Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study)
- PMID: 23240761
- PMCID: PMC3609903
- DOI: 10.1111/bjh.12163
Prediction and prevention of thromboembolic events with enoxaparin in cancer patients with elevated tissue factor-bearing microparticles: a randomized-controlled phase II trial (the Microtec study)
Abstract
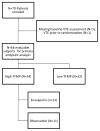
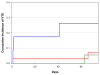
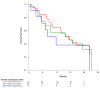
Elevated levels of circulating tissue factor-bearing microparticles (TFMP) have been associated with an increased risk of developing venous thromboembolism (VTE) in cancer patients. We performed a randomized phase II study to evaluate the cumulative incidence of VTE in advanced cancer patients with lower levels of TFMP not receiving thromboprophylaxis and those with higher levels of circulating TFMP randomized to enoxaparin or observation. The cumulative incidence of VTE at 2 months in the higher TFMP group randomized to enoxaparin (N = 23) was 5·6% while the higher TFMP group observation arm (N = 11) was 27·3% (Gray test P = 0·06). The cumulative incidence of VTE in the low TFMP was 7·2% (N = 32). No major haemorrhages were observed in the enoxaparin arm. The median survival for patients with higher levels of TFMP followed by observation was 11·8 months compared with 17·8 months on enoxaparin (P = 0·58). In a prospective randomized trial, increased numbers of circulating TFMP detected by impedance flow cytometry identified cancer patients with a high incidence of VTE. Enoxaparin demonstrated a clear trend towards reducing the rate of VTE in patients with elevated levels of TFMP, with an overall rate of VTE similar in magnitude to the lower TFMP group.
© 2012 Blackwell Publishing Ltd.
Conflict of interest statement
Conflict-of-interest disclosure: HAL has served on steering committees for Sanofi; CMK has received research funds and served on advisory boards for Sanofi and Esai. No other authors report relevant conflicts-of-interest.
Figures

Similar articles
-
Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy.Clin Cancer Res. 2009 Nov 15;15(22):6830-40. doi: 10.1158/1078-0432.CCR-09-0371. Epub 2009 Oct 27. Clin Cancer Res. 2009. PMID: 19861441 Free PMC article.
-
Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors.J Thromb Thrombolysis. 2020 Jul;50(1):211-216. doi: 10.1007/s11239-020-02146-z. J Thromb Thrombolysis. 2020. PMID: 32451823 Free PMC article.
-
Predictive value of tissue factor bearing microparticles in cancer associated thrombosis.Thromb Res. 2010 Apr;125 Suppl 2:S89-91. doi: 10.1016/S0049-3848(10)70022-0. Thromb Res. 2010. PMID: 20434015 Review.
-
Ximelagatran/Melagatran: a review of its use in the prevention of venous thromboembolism in orthopaedic surgery.Drugs. 2004;64(6):649-78. doi: 10.2165/00003495-200464060-00010. Drugs. 2004. PMID: 15018597 Review.
-
Heparin versus enoxaparin for prevention of venous thromboembolism after trauma: A randomized noninferiority trial.J Trauma Acute Care Surg. 2015 Dec;79(6):961-8; discussion 968-9. doi: 10.1097/TA.0000000000000750. J Trauma Acute Care Surg. 2015. PMID: 26317819 Clinical Trial.
Cited by
-
Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer.JCI Insight. 2019 Feb 21;4(4):e125851. doi: 10.1172/jci.insight.125851. eCollection 2019 Feb 21. JCI Insight. 2019. PMID: 30652973 Free PMC article. Clinical Trial.
-
White blood cell count measured prior to cancer development is associated with future risk of venous thromboembolism--the Tromsø study.PLoS One. 2013 Sep 4;8(9):e73447. doi: 10.1371/journal.pone.0073447. eCollection 2013. PLoS One. 2013. PMID: 24023876 Free PMC article.
-
Updated meta-analysis on prevention of venous thromboembolism in ambulatory cancer patients.Haematologica. 2020 Mar;105(3):838-848. doi: 10.3324/haematol.2019.221424. Epub 2019 Jun 6. Haematologica. 2020. PMID: 31171643 Free PMC article.
-
Plasma tissue factor activity in lung cancer patients predicts venous thromboembolism and poor overall survival.Res Pract Thromb Haemost. 2024 Mar 1;8(2):102359. doi: 10.1016/j.rpth.2024.102359. eCollection 2024 Feb. Res Pract Thromb Haemost. 2024. PMID: 38666062 Free PMC article.
-
Differential biomarker profiles between unprovoked venous thromboembolism and cancer.Ann Med. 2020 Sep;52(6):310-320. doi: 10.1080/07853890.2020.1779956. Epub 2020 Jul 15. Ann Med. 2020. PMID: 32634035 Free PMC article.
References
-
- Agnelli G, Gussoni G, Bianchini C, Verso M, Mandala M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G, Passalacqua R, Ricci S, Gasparini G, Lorusso V, Bonizzoni E, Tonato M. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10:943–949. - PubMed
-
- Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, Mouret P, Chaudhari U, Lawson F, Turpie AG. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601–609. - PubMed
-
- Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27:4124–4129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

