Kaposi's sarcoma-associated herpesvirus oncoprotein K13 protects against B cell receptor-induced growth arrest and apoptosis through NF-κB activation
- PMID: 23236068
- PMCID: PMC3571451
- DOI: 10.1128/JVI.01393-12
Kaposi's sarcoma-associated herpesvirus oncoprotein K13 protects against B cell receptor-induced growth arrest and apoptosis through NF-κB activation
Abstract
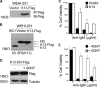
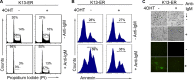
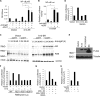
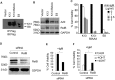
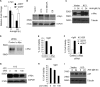
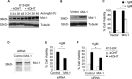
Kaposi's sarcoma-associated herpesvirus (KSHV) has been linked to the development of Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease (MCD). We have characterized the role of KSHV-encoded viral FLICE inhibitory protein (vFLIP) K13 in the modulation of anti-IgM-induced growth arrest and apoptosis in B cells. We demonstrate that K13 protects WEHI 231, an immature B-cell line, against anti-IgM-induced growth arrest and apoptosis. The protective effect of K13 was associated with the activation of the NF-κB pathway and was deficient in a mutant K13 with three alanine substitutions at positions 58 to 60 (K13-58AAA) and a structural homolog, vFLIP E8, both of which lack NF-κB activity. K13 upregulated the expression of NF-κB subunit RelB and blocked the anti-IgM-induced decline in c-Myc and rise in p27(Kip1) that have been associated with growth arrest and apoptosis. K13 also upregulated the expression of Mcl-1, an antiapoptotic member of the Bcl2 family. Finally, K13 protected the mature B-cell line Ramos against anti-IgM-induced apoptosis through NF-κB activation. Inhibition of anti-IgM-induced apoptosis by K13 may contribute to the development of KSHV-associated lymphoproliferative disorders.
Figures

Similar articles
-
NEMO is essential for Kaposi's sarcoma-associated herpesvirus-encoded vFLIP K13-induced gene expression and protection against death receptor-induced cell death, and its N-terminal 251 residues are sufficient for this process.J Virol. 2014 Jun;88(11):6345-54. doi: 10.1128/JVI.00028-14. Epub 2014 Mar 26. J Virol. 2014. PMID: 24672029 Free PMC article.
-
Nm23-H1 induces apoptosis in primary effusion lymphoma cells via inhibition of NF-κB signaling through interaction with oncogenic latent protein vFLIP K13 of Kaposi's sarcoma-associated herpes virus.Cell Oncol (Dordr). 2022 Oct;45(5):967-989. doi: 10.1007/s13402-022-00701-9. Epub 2022 Aug 14. Cell Oncol (Dordr). 2022. PMID: 35964258
-
A20 is induced by Kaposi sarcoma-associated herpesvirus-encoded viral FLICE inhibitory protein (vFLIP) K13 and blocks K13-induced nuclear factor-kappaB in a negative feedback manner.J Biol Chem. 2011 Jun 17;286(24):21555-64. doi: 10.1074/jbc.M111.224048. Epub 2011 Apr 29. J Biol Chem. 2011. PMID: 21531730 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus (KSHV/HHV8): key aspects of epidemiology and pathogenesis.AIDS Rev. 2003 Oct-Dec;5(4):222-9. AIDS Rev. 2003. PMID: 15012001 Review.
-
Pathological Features of Kaposi's Sarcoma-Associated Herpesvirus Infection.Adv Exp Med Biol. 2018;1045:357-376. doi: 10.1007/978-981-10-7230-7_16. Adv Exp Med Biol. 2018. PMID: 29896675 Review.
Cited by
-
KSHV Latency Locus Cooperates with Myc to Drive Lymphoma in Mice.PLoS Pathog. 2015 Sep 1;11(9):e1005135. doi: 10.1371/journal.ppat.1005135. eCollection 2015 Sep. PLoS Pathog. 2015. PMID: 26327622 Free PMC article.
-
BH3 Profiling Reveals Selectivity by Herpesviruses for Specific Bcl-2 Proteins To Mediate Survival of Latently Infected Cells.J Virol. 2015 May;89(10):5739-46. doi: 10.1128/JVI.00236-15. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740993 Free PMC article.
-
Mapping herpesvirus-driven impacts on the cellular milieu and transcriptional profile of Kaposi sarcoma in patient-derived mouse models.bioRxiv [Preprint]. 2024 Sep 28:2024.09.27.615429. doi: 10.1101/2024.09.27.615429. bioRxiv. 2024. PMID: 39386738 Free PMC article. Preprint.
-
Suppression of the SAP18/HDAC1 complex by targeting TRIM56 and Nanog is essential for oncogenic viral FLICE-inhibitory protein-induced acetylation of p65/RelA, NF-κB activation, and promotion of cell invasion and angiogenesis.Cell Death Differ. 2019 Oct;26(10):1970-1986. doi: 10.1038/s41418-018-0268-3. Epub 2019 Jan 22. Cell Death Differ. 2019. PMID: 30670829 Free PMC article.
-
Towards Better Understanding of KSHV Life Cycle: from Transcription and Posttranscriptional Regulations to Pathogenesis.Virol Sin. 2019 Apr;34(2):135-161. doi: 10.1007/s12250-019-00114-3. Epub 2019 Apr 25. Virol Sin. 2019. PMID: 31025296 Free PMC article. Review.
References
-
- Niiro H, Clark EA. 2002. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2:945–956 - PubMed
-
- Eeva J, Pelkonen J. 2004. Mechanisms of B cell receptor induced apoptosis. Apoptosis 9:525–531 - PubMed
-
- Gerondakis S, Siebenlist U. 2010. Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harbor Perspect. Biol. 2:a000182 doi:10.1101/cshperspect.a000182 - DOI - PMC - PubMed
-
- Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869 - PubMed
-
- Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. 1996. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma-associated herpes virus. Blood 88:645–656 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

