ARHGAP21 protein, a new partner of α-tubulin involved in cell-cell adhesion formation and essential for epithelial-mesenchymal transition
- PMID: 23235160
- PMCID: PMC3554890
- DOI: 10.1074/jbc.M112.432716
ARHGAP21 protein, a new partner of α-tubulin involved in cell-cell adhesion formation and essential for epithelial-mesenchymal transition
Abstract
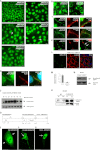
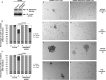
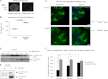
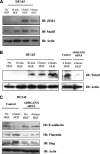
Cell-cell adhesions and the cytoskeletons play important and coordinated roles in cell biology, including cell differentiation, development, and migration. Adhesion and cytoskeletal dynamics are regulated by Rho-GTPases. ARHGAP21 is a negative regulator of Rho-GTPases, particularly Cdc42. Here we assess the function of ARHGAP21 in cell-cell adhesion, cell migration, and scattering. We find that ARHGAP21 is localized in the nucleus, cytoplasm, or perinuclear region but is transiently redistributed to cell-cell junctions 4 h after initiation of cell-cell adhesion. ARHGAP21 interacts with Cdc42, and decreased Cdc42 activity coincides with the appearance of ARHGAP21 at the cell-cell junctions. Cells lacking ARHGAP21 expression show weaker cell-cell adhesions, increased cell migration, and a diminished ability to undergo hepatocyte growth factor-induced epithelial-mesenchymal transition (EMT). In addition, ARHGAP21 interacts with α-tubulin, and it is essential for α-tubulin acetylation in EMT. Our findings indicate that ARHGAP21 is a Rho-GAP involved in cell-cell junction remodeling and that ARHGAP21 affects migration and EMT through α-tubulin interaction and acetylation.
Figures


Similar articles
-
ARHGAP21 modulates FAK activity and impairs glioblastoma cell migration.Biochim Biophys Acta. 2009 May;1793(5):806-16. doi: 10.1016/j.bbamcr.2009.02.010. Epub 2009 Mar 4. Biochim Biophys Acta. 2009. PMID: 19268501
-
Alpha-tubulin acetyltransferase/MEC-17 regulates cancer cell migration and invasion through epithelial-mesenchymal transition suppression and cell polarity disruption.Sci Rep. 2018 Nov 30;8(1):17477. doi: 10.1038/s41598-018-35392-6. Sci Rep. 2018. PMID: 30504808 Free PMC article.
-
Transport of influenza virus neuraminidase (NA) to host cell surface is regulated by ARHGAP21 and Cdc42 proteins.J Biol Chem. 2012 Mar 23;287(13):9804-9816. doi: 10.1074/jbc.M111.312959. Epub 2012 Feb 8. J Biol Chem. 2012. PMID: 22318733 Free PMC article.
-
Epithelial junctions and Rho family GTPases: the zonular signalosome.Small GTPases. 2014;5(4):1-15. doi: 10.4161/21541248.2014.973760. Small GTPases. 2014. PMID: 25483301 Free PMC article. Review.
-
ARHGAP21 as a master regulator of multiple cellular processes.J Cell Physiol. 2018 Nov;233(11):8477-8481. doi: 10.1002/jcp.26829. Epub 2018 Jun 1. J Cell Physiol. 2018. PMID: 29856495 Review.
Cited by
-
Prefoldin 1 promotes EMT and lung cancer progression by suppressing cyclin A expression.Oncogene. 2017 Feb 16;36(7):885-898. doi: 10.1038/onc.2016.257. Epub 2016 Oct 3. Oncogene. 2017. PMID: 27694898 Free PMC article.
-
Differential Expression of a Classic Cadherin Directs Tissue-Level Contractile Asymmetry during Neural Tube Closure.Dev Cell. 2019 Oct 21;51(2):158-172.e4. doi: 10.1016/j.devcel.2019.10.001. Dev Cell. 2019. PMID: 31639367 Free PMC article.
-
RNA-seq analysis identifies cytoskeletal structural genes and pathways for meat quality in beef.PLoS One. 2020 Nov 11;15(11):e0240895. doi: 10.1371/journal.pone.0240895. eCollection 2020. PLoS One. 2020. PMID: 33175867 Free PMC article.
-
The Actin Cytoskeleton Mediates Transmission of "Candidatus Liberibacter solanacearum" by the Carrot Psyllid.Appl Environ Microbiol. 2021 Jan 15;87(3):e02393-20. doi: 10.1128/AEM.02393-20. Print 2021 Jan 15. Appl Environ Microbiol. 2021. PMID: 33188004 Free PMC article.
-
Genetic Determinants of Electrocardiographic P-Wave Duration and Relation to Atrial Fibrillation.Circ Genom Precis Med. 2020 Oct;13(5):387-395. doi: 10.1161/CIRCGEN.119.002874. Epub 2020 Aug 21. Circ Genom Precis Med. 2020. PMID: 32822252 Free PMC article.
References
-
- Arthur W. T., Noren N. K., Burridge K. (2002) Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol. Res. 35, 239–246 - PubMed
-
- Fukata M., Kaibuchi K. (2001) Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat. Rev. Mol. Cell Biol. 2, 887–897 - PubMed
-
- Kim S. H., Li Z., Sacks D. B. (2000) E-cadherin-mediated cell-cell attachment activates Cdc42. J. Biol. Chem. 275, 36999–37005 - PubMed
-
- Van Aelst L., Symons M. (2002) Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 16, 1032–1054 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

