Chemoattractant receptor homologous to the T helper 2 cell (CRTH2) is not expressed in human amniocytes and myocytes
- PMID: 23226366
- PMCID: PMC3511345
- DOI: 10.1371/journal.pone.0050734
Chemoattractant receptor homologous to the T helper 2 cell (CRTH2) is not expressed in human amniocytes and myocytes
Abstract
Background: 15-deoxy-Δ 12,14- Prostaglandin J2 (15dPGJ2) inhibits Nuclear factor kappa B (NF-κB) in human myocytes and amniocytes and delays inflammation induced preterm labour in the mouse. 15dPGJ2 is a ligand for the Chemoattractant Receptor Homologous to the T helper 2 cell (CRTH2), a G protein-coupled receptor, present on a subset of T helper 2 (Th2) cells, eosinophils and basophils. It is the second receptor for Prostaglandin D2, whose activation leads to chemotaxis and the production of Th2-type interleukins. The cellular distribution of CRTH2 in non-immune cells has not been extensively researched, and its identification at the protein level has been limited by the lack of specific antibodies. In this study we explored the possibility that CRTH2 plays a role in 15dPGJ2-mediated inhibition of NF-κB and would therefore represent a novel small molecule therapeutic target for the prevention of inflammation induced preterm labour.
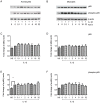
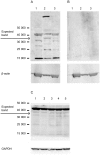
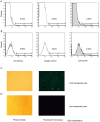
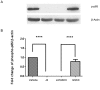
Methods: The effect of a small molecule CRTH2 agonist on NF-κB activity in human cultured amniocytes and myocytes was assessed by detection of p65 and phospho-p65 by immunoblot. Endogenous CRTH2 expression in amniocytes, myocytes and peripheral blood mononuclear cells (PBMCs) was examined by PCR, western analysis and flow cytometry, with amniocytes and myocytes transfected with CRTH2 acting as a positive control in flow cytometry studies.
Results: The CRTH2 agonist had no effect on NF-κB activity in amniocytes and myocytes. Although CRTH2 mRNA was detected in amniocytes and myocytes, CRTH2 was not detectable at the protein level, as demonstrated by western analysis and flow cytometry. 15dPGJ2 inhibited phospho-65 in PBMC'S, however the CRTH2 antagonist was not able to attenuate this effect. In conclusion, CRTH2 is not expressed on human amniocytes or myocytes and plays no role in the mechanism of 15dPGJ2-mediated inhibition of NF-κB.
Conflict of interest statement
Figures


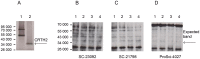

Similar articles
-
The CRTH2 agonist Pyl A prevents lipopolysaccharide-induced fetal death but induces preterm labour.Immunology. 2013 Jul;139(3):352-65. doi: 10.1111/imm.12085. Immunology. 2013. PMID: 23374103 Free PMC article.
-
Changes in the Th1:Th2 cytokine bias in pregnancy and the effects of the anti-inflammatory cyclopentenone prostaglandin 15-deoxy-Δ(12,14)-prostaglandin J2.Mediators Inflamm. 2012;2012:416739. doi: 10.1155/2012/416739. Epub 2012 May 29. Mediators Inflamm. 2012. PMID: 22690041 Free PMC article.
-
Differential modulation of human basophil functions through prostaglandin D2 receptors DP and chemoattractant receptor-homologous molecule expressed on Th2 cells/DP2.Clin Exp Allergy. 2004 Aug;34(8):1283-90. doi: 10.1111/j.1365-2222.2004.02027.x. Clin Exp Allergy. 2004. PMID: 15298571
-
The second PGD(2) receptor CRTH2: structure, properties, and functions in leukocytes.Prostaglandins Leukot Essent Fatty Acids. 2003 Aug-Sep;69(2-3):169-77. doi: 10.1016/s0952-3278(03)00078-4. Prostaglandins Leukot Essent Fatty Acids. 2003. PMID: 12895600 Review.
-
Antagonists of the prostaglandin D2 receptor CRTH2.Drug News Perspect. 2008 Jul-Aug;21(6):317-22. doi: 10.1358/dnp.2008.21.6.1246831. Drug News Perspect. 2008. PMID: 18836589 Review.
Cited by
-
15-Deoxy-Delta-12,14-prostaglandin J2 modulates pro-labour and pro-inflammatory responses in human myocytes, vaginal and amnion epithelial cells.Front Endocrinol (Lausanne). 2022 Sep 21;13:983924. doi: 10.3389/fendo.2022.983924. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36213265 Free PMC article.
-
Differential Response of Gestational Tissues to TLR3 Viral Priming Prior to Exposure to Bacterial TLR2 and TLR2/6 Agonists.Front Immunol. 2020 Aug 25;11:1899. doi: 10.3389/fimmu.2020.01899. eCollection 2020. Front Immunol. 2020. PMID: 32983111 Free PMC article.
-
The CRTH2 agonist Pyl A prevents lipopolysaccharide-induced fetal death but induces preterm labour.Immunology. 2013 Jul;139(3):352-65. doi: 10.1111/imm.12085. Immunology. 2013. PMID: 23374103 Free PMC article.
References
-
- Romero R, Mazor M, Munoz H, Gomez R, Galasso M, et al. (1994) The preterm labor syndrome. Ann N Y Acad Sci 734: 414–429. - PubMed
-
- Goldenberg RL, Hauth JC, Andrews WW (2000) Intrauterine infection and preterm delivery. N Engl J Med 342: 1500–1507. - PubMed
-
- Sykes L, MacIntyre D, Teoh T, Bennett P (2011) Targeting immune activation in the prevention of preterm labour. European Obstetrics and Gynaecology 6: 100–106.
-
- Lappas M, Rice GE (2009) Transcriptional regulation of the processes of human labour and delivery. Placenta 30 Suppl A: S90–95. - PubMed

