Adult cardiac progenitor cell aggregates exhibit survival benefit both in vitro and in vivo
- PMID: 23226295
- PMCID: PMC3511575
- DOI: 10.1371/journal.pone.0050491
Adult cardiac progenitor cell aggregates exhibit survival benefit both in vitro and in vivo
Abstract
Background: A major hurdle in the use of exogenous stems cells for therapeutic regeneration of injured myocardium remains the poor survival of implanted cells. To date, the delivery of stem cells into myocardium has largely focused on implantation of cell suspensions.
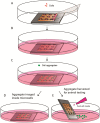
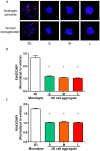
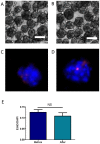
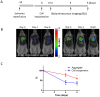
Methodology and principal findings: We hypothesize that delivering progenitor cells in an aggregate form would serve to mimic the endogenous state with proper cell-cell contact, and may aid the survival of implanted cells. Microwell methodologies allow for the culture of homogenous 3D cell aggregates, thereby allowing cell-cell contact. In this study, we find that the culture of cardiac progenitor cells in a 3D cell aggregate augments cell survival and protects against cellular toxins and stressors, including hydrogen peroxide and anoxia/reoxygenation induced cell death. Moreover, using a murine model of cardiac ischemia-reperfusion injury, we find that delivery of cardiac progenitor cells in the form of 3D aggregates improved in vivo survival of implanted cells.
Conclusion: Collectively, our data support the notion that growth in 3D cellular systems and maintenance of cell-cell contact improves exogenous cell survival following delivery into myocardium. These approaches may serve as a strategy to improve cardiovascular cell-based therapies.
Conflict of interest statement
Figures

Similar articles
-
Spatiotemporal control of cell fate and cardiac differentiation.Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:5567-8. doi: 10.1109/IEMBS.2011.6091346. Annu Int Conf IEEE Eng Med Biol Soc. 2011. PMID: 22255600
-
Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells.Circ Res. 2008 May 9;102(9):1075-81. doi: 10.1161/CIRCRESAHA.107.161729. Epub 2008 Mar 20. Circ Res. 2008. PMID: 18356544
-
3D-model of adult cardiac stem cells promotes cardiac differentiation and resistance to oxidative stress.J Cell Biochem. 2008 Oct 1;105(2):612-23. doi: 10.1002/jcb.21862. J Cell Biochem. 2008. PMID: 18661483
-
Cardiac side population cells: moving toward the center stage in cardiac regeneration.Circ Res. 2012 May 11;110(10):1355-63. doi: 10.1161/CIRCRESAHA.111.243014. Circ Res. 2012. PMID: 22581921 Free PMC article. Review.
-
Resident cardiac progenitor cells: at the heart of regeneration.J Mol Cell Cardiol. 2011 Feb;50(2):296-303. doi: 10.1016/j.yjmcc.2010.07.006. Epub 2010 Jul 17. J Mol Cell Cardiol. 2011. PMID: 20643135 Review.
Cited by
-
The Role of Cardiac Side Population Cells in Cardiac Regeneration.Front Cell Dev Biol. 2016 Sep 13;4:102. doi: 10.3389/fcell.2016.00102. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27679798 Free PMC article. Review.
-
Human pluripotent stem cell-derived cardiomyocytes for heart regeneration, drug discovery and disease modeling: from the genetic, epigenetic, and tissue modeling perspectives.Stem Cell Res Ther. 2013 Aug 14;4(4):97. doi: 10.1186/scrt308. Stem Cell Res Ther. 2013. PMID: 23953772 Free PMC article. Review.
-
Guidelines for experimental models of myocardial ischemia and infarction.Am J Physiol Heart Circ Physiol. 2018 Apr 1;314(4):H812-H838. doi: 10.1152/ajpheart.00335.2017. Epub 2018 Jan 12. Am J Physiol Heart Circ Physiol. 2018. PMID: 29351451 Free PMC article.
-
Cardiac stem cells: biology and clinical applications.Antioxid Redox Signal. 2014 Nov 10;21(14):2002-17. doi: 10.1089/ars.2014.5875. Epub 2014 Apr 10. Antioxid Redox Signal. 2014. PMID: 24597850 Free PMC article. Review.
-
Priming of the Cells: Hypoxic Preconditioning for Stem Cell Therapy.Chin Med J (Engl). 2017 Oct 5;130(19):2361-2374. doi: 10.4103/0366-6999.215324. Chin Med J (Engl). 2017. PMID: 28937044 Free PMC article. Review.
References
-
- Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, et al. (1996) Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335: 1182–1189. - PubMed
-
- Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, et al. (1997) Apoptosis in the failing human heart. N Engl J Med 336: 1131–1141. - PubMed
-
- Ye Z, Zhou Y, Cai H, Tan W (2011) Myocardial regeneration: Roles of stem cells and hydrogels. Adv Drug Deliv Rev 63: 688–697. - PubMed
-
- Ptaszek LM, Mansour M, Ruskin JN, Chien KR (2012) Towards regenerative therapy for cardiac disease. Lancet 379: 933–942. - PubMed

