Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function
- PMID: 23224399
- PMCID: PMC3563762
- DOI: 10.1158/1078-0432.CCR-12-2024
Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function
Abstract
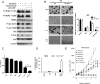
Purpose: Dual blockade of HER2 with trastuzumab and lapatinib or with pertuzumab is a superior treatment approach compared with single-agent HER2 inhibitors. However, many HER2-overexpressing breast cancers still escape from this combinatorial approach. Inhibition of HER2 and downstream phosphoinositide 3-kinase (PI3K)/AKT causes a transcriptional and posttranslational upregulation of HER3 which, in turn, counteracts the antitumor action of the HER2-directed therapies. We hypothesized that suppression of HER3 would synergize with dual blockade of HER2 in breast cancer cells sensitive and refractory to HER2 antagonists.
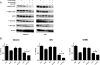
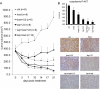
Experimental design: Inhibition of HER2/HER3 in HER2(+) breast cancer cell lines was evaluated by Western blotting. We analyzed drug-induced apoptosis and two- and three-dimensional growth in vitro. Growth inhibition of PI3K was examined in vivo in xenografts treated with combinations of trastuzumab, lapatinib, and the HER3-neutralizing monoclonal antibody U3-1287.
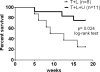
Results: Treatment with U3-1287 blocked the upregulation of total and phosphorylated HER3 that followed treatment with lapatinib and trastuzumab and, in turn, enhanced the antitumor action of the combination against trastuzumab-sensitive and -resistant cells. Mice bearing HER2(+) xenografts treated with lapatinib, trastuzumab, and U3-1287 exhibited fewer recurrences and better survival than mice treated with lapatinib and trastuzumab.
Conclusions: Dual blockade of HER2 with trastuzumab and lapatinib does not eliminate the compensatory upregulation of HER3. Therapeutic inhibitors of HER3 should be considered as part of multidrug combinations aimed at completely and rapidly disabling the HER2 network in HER2-overexpressing breast cancers.
Figures

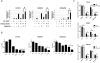
Similar articles
-
Combination of antibody that inhibits ligand-independent HER3 dimerization and a p110α inhibitor potently blocks PI3K signaling and growth of HER2+ breast cancers.Cancer Res. 2013 Oct 1;73(19):6013-23. doi: 10.1158/0008-5472.CAN-13-1191. Epub 2013 Aug 5. Cancer Res. 2013. PMID: 23918797 Free PMC article.
-
Dual mTORC1/2 and HER2 blockade results in antitumor activity in preclinical models of breast cancer resistant to anti-HER2 therapy.Clin Cancer Res. 2012 May 1;18(9):2603-12. doi: 10.1158/1078-0432.CCR-11-2750. Epub 2012 Mar 8. Clin Cancer Res. 2012. PMID: 22407832
-
Sustained Inhibition of HER3 and EGFR Is Necessary to Induce Regression of HER2-Amplified Gastrointestinal Carcinomas.Clin Cancer Res. 2015 Dec 15;21(24):5519-31. doi: 10.1158/1078-0432.CCR-14-3066. Epub 2015 Aug 21. Clin Cancer Res. 2015. PMID: 26296355
-
Lapatinib.Recent Results Cancer Res. 2018;211:19-44. doi: 10.1007/978-3-319-91442-8_2. Recent Results Cancer Res. 2018. PMID: 30069757 Review.
-
A systematic review of dual targeting in HER2-positive breast cancer.Cancer Treat Rev. 2014 Mar;40(2):259-70. doi: 10.1016/j.ctrv.2013.09.002. Epub 2013 Sep 11. Cancer Treat Rev. 2014. PMID: 24080156 Review.
Cited by
-
Anti-tumoral activity of the Pan-HER (Sym013) antibody mixture in gemcitabine-resistant pancreatic cancer models.MAbs. 2021 Jan-Dec;13(1):1914883. doi: 10.1080/19420862.2021.1914883. MAbs. 2021. PMID: 33876707 Free PMC article.
-
A novel mechanism of action of HER2 targeted immunotherapy is explained by inhibition of NRF2 function in ovarian cancer cells.Oncotarget. 2016 Nov 15;7(46):75874-75901. doi: 10.18632/oncotarget.12425. Oncotarget. 2016. PMID: 27713148 Free PMC article.
-
Lapatinib resistance in HER2+ cancers: latest findings and new concepts on molecular mechanisms.Tumour Biol. 2016 Oct 10. doi: 10.1007/s13277-016-5467-2. Online ahead of print. Tumour Biol. 2016. PMID: 27726101 Review.
-
Risk of severe diarrhea with dual anti-HER2 therapies: a meta-analysis.Tumour Biol. 2014 May;35(5):4077-85. doi: 10.1007/s13277-013-1533-1. Epub 2013 Dec 29. Tumour Biol. 2014. PMID: 24375251
-
Dual HER2 blockade in the neoadjuvant and adjuvant treatment of HER2-positive breast cancer.Breast Cancer (Dove Med Press). 2015 Sep 22;7:321-35. doi: 10.2147/BCTT.S90627. eCollection 2015. Breast Cancer (Dove Med Press). 2015. PMID: 26451122 Free PMC article. Review.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. - PubMed
-
- Press MF, Bernstein L, Thomas PA, Meisner LF, Zhou JY, Ma Y, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15(8):2894–904. - PubMed
-
- Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–40. - PubMed
-
- Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–96. - PubMed
-
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets [see comments]. Nat Med. 2000;6(4):443–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

