Arl13b regulates endocytic recycling traffic
- PMID: 23223633
- PMCID: PMC3535586
- DOI: 10.1073/pnas.1218272110
Arl13b regulates endocytic recycling traffic
Abstract
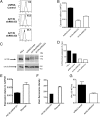
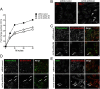
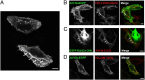
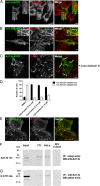
Intracellular recycling pathways play critical roles in internalizing membrane and fluid phase cargo and in balancing the inflow and outflow of membrane and cell surface molecules. To identify proteins involved in the regulation of endocytic recycling, we used an shRNA trafficking library and screened for changes in the surface expression of CD1a antigen-presenting molecules that follow an endocytic recycling route. We found that silencing of the ADP-ribosylation factor (Arf)-like small GTPase Arl13b led to a decrease in CD1a surface expression, diminished CD1a function, and delayed CD1a recycling, suggesting that Arl13b is involved in the regulation of endocytic recycling traffic. Arl13b appears to be required for the major route of endocytic trafficking, causing clustering of early endosomes and leading to the accumulation of endocytic cargo. Moreover, Arl13b colocalized with markers of the endocytic recycling pathway followed by CD1a, namely Arf6 and Rab22a. We also detected an interaction between Arl13b and the actin cytoskeleton. Arl13b was previously implicated in cilia formation and function. Our present results indicate a previously unidentified role for Arl13b in endocytic recycling traffic and suggest a link between Arl13b function and the actin cytoskeleton.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
CD1a and MHC class I follow a similar endocytic recycling pathway.Traffic. 2008 Sep;9(9):1446-57. doi: 10.1111/j.1600-0854.2008.00781.x. Epub 2008 Jun 28. Traffic. 2008. PMID: 18564371 Free PMC article.
-
CD1a molecules traffic through the early recycling endosomal pathway in human Langerhans cells.J Invest Dermatol. 2001 Mar;116(3):401-8. doi: 10.1046/j.1523-1747.2001.01264.x. J Invest Dermatol. 2001. PMID: 11231314
-
Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome.Mol Biol Cell. 2008 Oct;19(10):4224-37. doi: 10.1091/mbc.e08-03-0290. Epub 2008 Aug 6. Mol Biol Cell. 2008. PMID: 18685082 Free PMC article.
-
ARF6-mediated endocytic recycling impacts cell movement, cell division and lipid homeostasis.Semin Cell Dev Biol. 2011 Feb;22(1):39-47. doi: 10.1016/j.semcdb.2010.09.002. Epub 2010 Sep 15. Semin Cell Dev Biol. 2011. PMID: 20837153 Free PMC article. Review.
-
Rab35 GTPase: A Central Regulator of Phosphoinositides and F-actin in Endocytic Recycling and Beyond.Traffic. 2016 Oct;17(10):1063-77. doi: 10.1111/tra.12422. Epub 2016 Jul 14. Traffic. 2016. PMID: 27329675 Review.
Cited by
-
Primary cilia found on HeLa and other cancer cells.Cell Biol Int. 2015 Nov;39(11):1341-7. doi: 10.1002/cbin.10500. Epub 2015 Aug 6. Cell Biol Int. 2015. PMID: 26074404 Free PMC article.
-
Ciliary transport regulates PDGF-AA/αα signaling via elevated mammalian target of rapamycin signaling and diminished PP2A activity.Mol Biol Cell. 2015 Jan 15;26(2):350-8. doi: 10.1091/mbc.E14-05-0952. Epub 2014 Nov 12. Mol Biol Cell. 2015. PMID: 25392303 Free PMC article.
-
The Role of ARF Family Proteins and Their Regulators and Effectors in Cancer Progression: A Therapeutic Perspective.Front Cell Dev Biol. 2020 Apr 21;8:217. doi: 10.3389/fcell.2020.00217. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32426352 Free PMC article. Review.
-
ARL13B regulates Sonic hedgehog signaling from outside primary cilia.Elife. 2020 Mar 4;9:e50434. doi: 10.7554/eLife.50434. Elife. 2020. PMID: 32129762 Free PMC article.
-
Vesicular Trafficking to the Immune Synapse: How to Assemble Receptor-Tailored Pathways from a Basic Building Set.Front Immunol. 2016 Feb 15;7:50. doi: 10.3389/fimmu.2016.00050. eCollection 2016. Front Immunol. 2016. PMID: 26913036 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

