A dominant mutation in mec-7/β-tubulin affects axon development and regeneration in Caenorhabditis elegans neurons
- PMID: 23223572
- PMCID: PMC3564523
- DOI: 10.1091/mbc.E12-06-0441
A dominant mutation in mec-7/β-tubulin affects axon development and regeneration in Caenorhabditis elegans neurons
Abstract
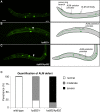
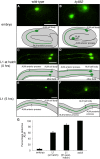
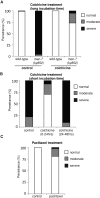
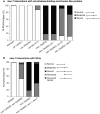
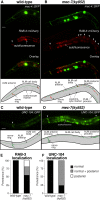
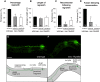
Microtubules have been known for decades to be basic elements of the cytoskeleton. They form long, dynamic, rope-like structures within the cell that are essential for mitosis, maintenance of cell shape, and intracellular transport. More recently, in vitro studies have implicated microtubules as signaling molecules that, through changes in their stability, have the potential to trigger growth of axons and dendrites in developing neurons. In this study, we show that specific mutations in the Caenorhabditis elegans mec-7/β-tubulin gene cause ectopic axon formation in mechanosensory neurons in vivo. In mec-7 mutants, the ALM mechanosensory neuron forms a long ectopic neurite that extends posteriorly, a phenotype that can be mimicked in wild-type worms with a microtubule-stabilizing drug (paclitaxel), and suppressed by mutations in unc-33/CRMP2 and the kinesin-related gene, vab-8. Our results also reveal that these ectopic neurites contain RAB-3, a marker for presynaptic loci, suggesting that they have axon-like properties. Interestingly, in contrast with the excessive axonal growth observed during development, mec-7 mutants are inhibited in axonal regrowth and remodeling following axonal injury. Together our results suggest that MEC-7/β-tubulin integrity is necessary for the correct number of neurites a neuron generates in vivo and for the capacity of an axon to regenerate.
Figures

Similar articles
-
RHGF-1/PDZ-RhoGEF and retrograde DLK-1 signaling drive neuronal remodeling on microtubule disassembly.Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16568-73. doi: 10.1073/pnas.1410263111. Epub 2014 Oct 30. Proc Natl Acad Sci U S A. 2014. PMID: 25359212 Free PMC article.
-
The multipurpose 15-protofilament microtubules in C. elegans have specific roles in mechanosensation.Curr Biol. 2009 Aug 25;19(16):1362-7. doi: 10.1016/j.cub.2009.06.036. Epub 2009 Jul 16. Curr Biol. 2009. PMID: 19615905 Free PMC article.
-
The atRA-responsive gene neuron navigator 2 functions in neurite outgrowth and axonal elongation.Dev Neurobiol. 2008 Nov;68(13):1441-53. doi: 10.1002/dneu.20670. Dev Neurobiol. 2008. PMID: 18726912 Free PMC article.
-
Unraveling the mechanisms of synapse formation and axon regeneration: the awesome power of C. elegans genetics.Sci China Life Sci. 2015 Nov;58(11):1084-8. doi: 10.1007/s11427-015-4962-9. Epub 2015 Nov 13. Sci China Life Sci. 2015. PMID: 26563175 Free PMC article. Review.
-
The Genetics of Axon Guidance and Axon Regeneration in Caenorhabditis elegans.Genetics. 2016 Nov;204(3):849-882. doi: 10.1534/genetics.115.186262. Genetics. 2016. PMID: 28114100 Free PMC article. Review.
Cited by
-
Diapause induces functional axonal regeneration after necrotic insult in C. elegans.PLoS Genet. 2019 Jan 14;15(1):e1007863. doi: 10.1371/journal.pgen.1007863. eCollection 2019 Jan. PLoS Genet. 2019. PMID: 30640919 Free PMC article.
-
Epothilone B Speeds Corneal Nerve Regrowth and Functional Recovery through Microtubule Stabilization and Increased Nerve Beading.Sci Rep. 2018 Feb 8;8(1):2647. doi: 10.1038/s41598-018-20734-1. Sci Rep. 2018. PMID: 29422528 Free PMC article.
-
Loss of MEC-17 leads to microtubule instability and axonal degeneration.Cell Rep. 2014 Jan 16;6(1):93-103. doi: 10.1016/j.celrep.2013.12.004. Epub 2013 Dec 27. Cell Rep. 2014. PMID: 24373971 Free PMC article.
-
A multi-channel device for high-density target-selective stimulation and long-term monitoring of cells and subcellular features in C. elegans.Lab Chip. 2014 Dec 7;14(23):4513-4522. doi: 10.1039/c4lc00789a. Epub 2014 Sep 26. Lab Chip. 2014. PMID: 25257026 Free PMC article.
-
Synaptic branch stability is mediated by non-enzymatic functions of MEC-17/αTAT1 and ATAT-2.Sci Rep. 2022 Aug 17;12(1):14003. doi: 10.1038/s41598-022-18333-2. Sci Rep. 2022. PMID: 35977998 Free PMC article.
References
-
- Baas PW. Neuronal polarity: microtubules strike back. Nat Cell Biol. 2002;4:E194–E195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

