Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells
- PMID: 23223361
- PMCID: PMC3587322
- DOI: 10.1182/blood-2012-09-457465
Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells
Abstract
Regulatory T cells (Tregs) play an essential role in preventing autoimmunity. Mutations in the forkhead box protein 3 (FOXP3) gene, which encodes a transcription factor critical for Treg function, result in a severe autoimmune disorder and the production of various autoantibodies in mice and in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) patients. However, it is unknown whether Tregs normally suppress autoreactive B cells. To investigate a role for Tregs in maintaining human B-cell tolerance, we tested the reactivity of recombinant antibodies isolated from single B cells isolated from IPEX patients. Characteristics and reactivity of antibodies expressed by new emigrant/transitional B cells from IPEX patients were similar to those from healthy donors, demonstrating that defective Treg function does not impact central B-cell tolerance. In contrast, mature naive B cells from IPEX patients often expressed autoreactive antibodies, suggesting an important role for Tregs in maintaining peripheral B-cell tolerance. T cells displayed an activated phenotype in IPEX patients, including their Treg-like cells, and showed up-regulation of CD40L, PD-1, and inducibl T-cell costimulator (ICOS), which may favor the accumulation of autoreactive mature naive B cells in these patients. Hence, our data demonstrate an essential role for Tregs in the establishment and the maintenance of peripheral B-cell tolerance in humans.
Figures


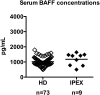
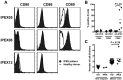

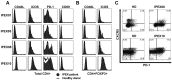
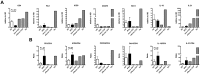
Similar articles
-
Identification of unstable regulatory and autoreactive effector T cells that are expanded in patients with FOXP3 mutations.Sci Transl Med. 2023 Dec 20;15(727):eadg6822. doi: 10.1126/scitranslmed.adg6822. Epub 2023 Dec 20. Sci Transl Med. 2023. PMID: 38117899 Free PMC article.
-
Anti-CD4 treatment inhibits autoimmunity in scurfy mice through the attenuation of co-stimulatory signals.J Autoimmun. 2014 May;50:23-32. doi: 10.1016/j.jaut.2013.08.010. Epub 2013 Sep 25. J Autoimmun. 2014. PMID: 24075450
-
Point mutants of forkhead box P3 that cause immune dysregulation, polyendocrinopathy, enteropathy, X-linked have diverse abilities to reprogram T cells into regulatory T cells.J Allergy Clin Immunol. 2010 Dec;126(6):1242-51. doi: 10.1016/j.jaci.2010.09.001. Epub 2010 Oct 30. J Allergy Clin Immunol. 2010. PMID: 21036387
-
Regulatory T cells in peripheral tissue tolerance and diseases.Front Immunol. 2023 May 1;14:1154575. doi: 10.3389/fimmu.2023.1154575. eCollection 2023. Front Immunol. 2023. PMID: 37197653 Free PMC article. Review.
-
[IPEX syndrome and human Treg cells].Nihon Rinsho Meneki Gakkai Kaishi. 2010;33(4):196-206. doi: 10.2177/jsci.33.196. Nihon Rinsho Meneki Gakkai Kaishi. 2010. PMID: 20818148 Review. Japanese.
Cited by
-
Safety and efficacy profile of mogamulizumab (Poteligeo) in the treatment of cancers: an update evidence from 14 studies.BMC Cancer. 2021 May 26;21(1):618. doi: 10.1186/s12885-021-08363-w. BMC Cancer. 2021. PMID: 34039310 Free PMC article.
-
Identification of unstable regulatory and autoreactive effector T cells that are expanded in patients with FOXP3 mutations.Sci Transl Med. 2023 Dec 20;15(727):eadg6822. doi: 10.1126/scitranslmed.adg6822. Epub 2023 Dec 20. Sci Transl Med. 2023. PMID: 38117899 Free PMC article.
-
Altered B cell signalling in autoimmunity.Nat Rev Immunol. 2017 Jul;17(7):421-436. doi: 10.1038/nri.2017.24. Epub 2017 Apr 10. Nat Rev Immunol. 2017. PMID: 28393923 Free PMC article. Review.
-
TNF receptor superfamily member 13b (TNFRSF13B) hemizygosity reveals transmembrane activator and CAML interactor haploinsufficiency at later stages of B-cell development.J Allergy Clin Immunol. 2015 Nov;136(5):1315-25. doi: 10.1016/j.jaci.2015.05.012. Epub 2015 Jun 19. J Allergy Clin Immunol. 2015. PMID: 26100089 Free PMC article.
-
Anti-cytokine autoantibodies: mechanistic insights and disease associations.Nat Rev Immunol. 2024 Mar;24(3):161-177. doi: 10.1038/s41577-023-00933-2. Epub 2023 Sep 19. Nat Rev Immunol. 2024. PMID: 37726402 Review.
References
-
- Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. - PubMed
-
- Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

