RNAi screening identifies mediators of NOD2 signaling: implications for spatial specificity of MDP recognition
- PMID: 23213202
- PMCID: PMC3535590
- DOI: 10.1073/pnas.1209673109
RNAi screening identifies mediators of NOD2 signaling: implications for spatial specificity of MDP recognition
Abstract
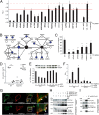
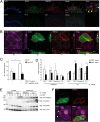
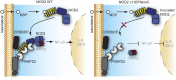
The intracellular nucleotide-binding oligomerization domain-2 (NOD2) receptor detects bacteria-derived muramyl dipeptide (MDP) and activates the transcription factor NF-κB. Here we describe the regulatome of NOD2 signaling using a systematic RNAi screen. Using three consecutive screens, we identified a set of 20 positive NF-κB regulators including the known pathway members RIPK2, RELA, and BIRC4 (XIAP) as well as FRMPD2 (FERM and PDZ domain-containing 2). FRMPD2 interacts with NOD2 via leucine-rich repeats and forms a complex with the membrane-associated protein ERBB2IP. We demonstrate that FRMPD2 spatially assembles the NOD2-signaling complex, hereby restricting NOD2-mediated immune responses to the basolateral compartment of polarized intestinal epithelial cells. We show that genetic truncation of the NOD2 leucine-rich repeat domain, which is associated with Crohn disease, impairs the interaction with FRMPD2, and that intestinal inflammation leads to down-regulation of FRMPD2. These results suggest a structural mechanism for how polarity of epithelial cells acts on intestinal NOD-like receptor signaling to mediate spatial specificity of bacterial recognition and control of immune responses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
FRMBP2 directs NOD2 to the membrane.Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21188-9. doi: 10.1073/pnas.1219395110. Epub 2012 Dec 17. Proc Natl Acad Sci U S A. 2012. PMID: 23248319 Free PMC article. No abstract available.
Similar articles
-
The NOD2-RICK complex signals from the plasma membrane.J Biol Chem. 2007 May 18;282(20):15197-207. doi: 10.1074/jbc.M606242200. Epub 2007 Mar 13. J Biol Chem. 2007. PMID: 17355968
-
Pathogen sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is mediated by direct binding to muramyl dipeptide and ATP.J Biol Chem. 2012 Jun 29;287(27):23057-67. doi: 10.1074/jbc.M112.344283. Epub 2012 May 1. J Biol Chem. 2012. PMID: 22549783 Free PMC article.
-
RIPK2 Is Crucial for the Microglial Inflammatory Response to Bacterial Muramyl Dipeptide but Not to Lipopolysaccharide.Int J Mol Sci. 2024 Nov 1;25(21):11754. doi: 10.3390/ijms252111754. Int J Mol Sci. 2024. PMID: 39519307 Free PMC article.
-
Muramyl dipeptide responsive pathways in Crohn's disease: from NOD2 and beyond.Cell Mol Life Sci. 2013 Sep;70(18):3391-404. doi: 10.1007/s00018-012-1246-4. Epub 2012 Dec 29. Cell Mol Life Sci. 2013. PMID: 23275943 Free PMC article. Review.
-
Nucleotide-binding oligomerization domain containing 2: structure, function, and diseases.Semin Arthritis Rheum. 2013 Aug;43(1):125-30. doi: 10.1016/j.semarthrit.2012.12.005. Epub 2013 Jan 24. Semin Arthritis Rheum. 2013. PMID: 23352252 Review.
Cited by
-
Membrane Association Dictates Ligand Specificity for the Innate Immune Receptor NOD2.ACS Chem Biol. 2017 Aug 18;12(8):2216-2224. doi: 10.1021/acschembio.7b00469. Epub 2017 Jul 25. ACS Chem Biol. 2017. PMID: 28708377 Free PMC article.
-
E3 Ubiquitin ligase ZNRF4 negatively regulates NOD2 signalling and induces tolerance to MDP.Nat Commun. 2017 Jun 28;8:15865. doi: 10.1038/ncomms15865. Nat Commun. 2017. PMID: 28656966 Free PMC article.
-
Detection of enteric pathogens by the nodosome.Trends Immunol. 2014 Mar;35(3):123-30. doi: 10.1016/j.it.2013.10.009. Epub 2013 Nov 22. Trends Immunol. 2014. PMID: 24268520 Free PMC article. Review.
-
A new functional assay for the diagnosis of X-linked inhibitor of apoptosis (XIAP) deficiency.Clin Exp Immunol. 2014 Jun;176(3):394-400. doi: 10.1111/cei.12306. Clin Exp Immunol. 2014. PMID: 24611904 Free PMC article.
-
NOD2 and inflammation: current insights.J Inflamm Res. 2018 Feb 12;11:49-60. doi: 10.2147/JIR.S137606. eCollection 2018. J Inflamm Res. 2018. PMID: 29483781 Free PMC article. Review.
References
-
- Maekawa T, Kufer TA, Schulze-Lefert P. NLR functions in plant and animal immune systems: So far and yet so close. Nat Immunol. 2011;12(9):817–826. - PubMed
-
- Lange C, et al. Defining the origins of the NOD-like receptor system at the base of animal evolution. Mol Biol Evol. 2011;28(5):1687–1702. - PubMed
-
- Ogura Y, et al. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276(7):4812–4818. - PubMed
-
- Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–8872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

