Dendritic cell modification as a route to inhibiting corneal graft rejection by the indirect pathway of allorecognition
- PMID: 23212959
- PMCID: PMC3615172
- DOI: 10.1002/eji.201242914
Dendritic cell modification as a route to inhibiting corneal graft rejection by the indirect pathway of allorecognition
Abstract
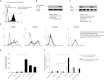
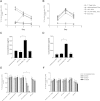
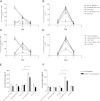
Dendritic cell (DC) modification is a potential strategy to induce clinical transplantation tolerance. We compared two DC modification strategies to inhibit allogeneic T-cell proliferation. In the first strategy, murine DCs were transduced with a lentiviral vector expressing CTLA4-KDEL, a fusion protein that prevents surface CD80/86 expression by retaining the co-stimulatory molecules within the ER. In the second approach, DCs were transduced to express the tryptophan-catabolising enzyme IDO. CTLA4-KDEL-expressing DCs induced anergy in alloreactive T cells and generated both CD4(+) CD25(+) and CD4(+) CD25(-) Treg cells (with direct and indirect donor allospecificity and capacity for linked suppression) both in vitro and in vivo. In contrast, T-cell unresponsiveness induced by IDO(+) DCs lacked donor specificity. In the absence of any immunosuppressive treatment, i.v. administration of CTLA4-KDEL-expressing DCs resulted in long-term survival of corneal allografts only when the DCs were capable of indirect presentation of alloantigen. This study demonstrates the therapeutic potential of CTLA4-KDEL-expressing DCs in tolerance induction.
© 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
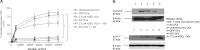


Comment in
-
The road to transplant tolerance is paved with good dendritic cells.Eur J Immunol. 2013 Mar;43(3):584-8. doi: 10.1002/eji.201343361. Eur J Immunol. 2013. PMID: 23412714 Free PMC article.
Similar articles
-
Generation of therapeutic dendritic cells and regulatory T cells for preventing allogeneic cardiac graft rejection.Clin Immunol. 2008 Jun;127(3):313-21. doi: 10.1016/j.clim.2008.01.013. Epub 2008 Mar 20. Clin Immunol. 2008. PMID: 18358783
-
Tolerogenic dendritic cells suppress murine corneal allograft rejection by modulating CD28/CTLA-4 expression on regulatory T cells.Cell Biol Int. 2014 Jul;38(7):835-48. doi: 10.1002/cbin.10268. Epub 2014 Mar 14. Cell Biol Int. 2014. PMID: 24604878
-
Expression of the chemokine decoy receptor D6 mediates dendritic cell function and promotes corneal allograft rejection.Mol Vis. 2013 Dec 16;19:2517-25. eCollection 2013. Mol Vis. 2013. PMID: 24357920 Free PMC article.
-
Aspirin and the induction of tolerance by dendritic cells.Handb Exp Pharmacol. 2009;(188):197-213. doi: 10.1007/978-3-540-71029-5_9. Handb Exp Pharmacol. 2009. PMID: 19031027 Review.
-
Manipulation of Regulatory Dendritic Cells for Induction Transplantation Tolerance.Front Immunol. 2020 Oct 14;11:582658. doi: 10.3389/fimmu.2020.582658. eCollection 2020. Front Immunol. 2020. PMID: 33162996 Free PMC article. Review.
Cited by
-
Therapeutic approaches for induction of tolerance and immune quiescence in corneal allotransplantation.Cell Mol Life Sci. 2018 May;75(9):1509-1520. doi: 10.1007/s00018-017-2739-y. Epub 2018 Jan 6. Cell Mol Life Sci. 2018. PMID: 29307015 Free PMC article. Review.
-
Contribution of dendritic cells to the autoimmune pathology of systemic lupus erythematosus.Immunology. 2015 Dec;146(4):497-507. doi: 10.1111/imm.12504. Epub 2015 Oct 12. Immunology. 2015. PMID: 26173489 Free PMC article. Review.
-
Aspects of Corneal Transplant Immunology.J Ophthalmic Vis Res. 2017 Jul-Sep;12(3):249-250. doi: 10.4103/jovr.jovr_115_17. J Ophthalmic Vis Res. 2017. PMID: 28791054 Free PMC article. No abstract available.
-
Donor bone marrow-derived dendritic cells prolong corneal allograft survival and promote an intragraft immunoregulatory milieu.Mol Ther. 2013 Nov;21(11):2102-12. doi: 10.1038/mt.2013.167. Epub 2013 Jul 18. Mol Ther. 2013. PMID: 23863882 Free PMC article.
-
Dendritic Cell-Induced Th1 and Th17 Cell Differentiation for Cancer Therapy.Vaccines (Basel). 2013 Nov 21;1(4):527-49. doi: 10.3390/vaccines1040527. Vaccines (Basel). 2013. PMID: 26344346 Free PMC article. Review.
References
-
- Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 2007;7:610–621. - PubMed
-
- Mirenda V, Berton I, Read J, Cook T, Smith J, Dorling A, Lechler RI. Modified dendritic cells coexpressing self and allogeneic major histocompatability complex molecules: an efficient way to induce indirect pathway regulation. J. Am. Soc. Nephrol. 2004;15:987–997. - PubMed
-
- Andreakos E, Smith C, Monaco C, Brennan FM, Foxwell BM, Feldmann M. Ikappa B kinase 2 but not NF-kappa B-inducing kinase is essential for effective DC antigen presentation in the allogeneic mixed lymphocyte reaction. Blood. 2003;101:983–991. - PubMed
-
- Yoshimura S, Bondeson J, Brennan FM, Foxwell BM, Feldmann M. Antigen presentation by murine dendritic cells is nuclear factor-kappa B dependent both in vitro and in vivo. Scand. J. Immunol. 2003;58:165–172. - PubMed
-
- Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

