Protection of mouse retinal ganglion cell axons and soma from glaucomatous and ischemic injury by cytoplasmic overexpression of Nmnat1
- PMID: 23211826
- PMCID: PMC3541947
- DOI: 10.1167/iovs.12-10861
Protection of mouse retinal ganglion cell axons and soma from glaucomatous and ischemic injury by cytoplasmic overexpression of Nmnat1
Abstract
Purpose: The Wlds mutation affords protection of retinal ganglion cell (RGC) axons in retinal ischemia and in inducible and hereditary preclinical models of glaucoma. We undertook the present study to determine whether the Nmnat1 portion of the chimeric protein provides axonal and somatic protection of RGCs in models of ischemia and glaucoma, particularly when localized to nonnuclear regions of the cell.
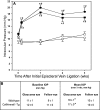
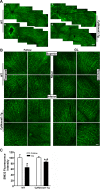
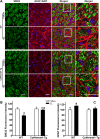
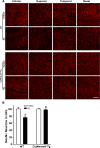
Methods: The survival and integrity of RGC axons and soma from transgenic mice with confirmed cytoplasmic overexpression of Nmnat1 in retina and optic nerve (cytNmnat1-Tg mice) were examined in the retina and postlaminar optic nerve 4 days following acute retinal ischemia, and 3 weeks following the chronic elevation of intraocular pressure.
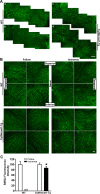
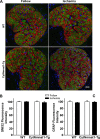
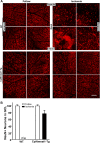
Results: Ischemia- and glaucoma-induced disruptions of proximal segments of RGC axons that comprise the nerve fiber layer in wild-type mice were both robustly abrogated in cytNmnat1-Tg mice. More distal portions of RGC axons within the optic nerve were also protected from glaucomatous disruption in the transgenic mice. In both disease models, Nmnat1 overexpression in extranuclear locations significantly enhanced the survival of RGC soma.
Conclusions: Overexpression of Nmnat1 in the cytoplasm and axons of RGCs robustly protected against both ischemic and glaucomatous loss of RGC axonal integrity, as well as loss of RGC soma. These findings reflect the more pan-cellular protection of CNS neurons that is realized by cytoplasmic Nmnat1 expression, and thus provide a therapeutic strategy for protecting against retinal neurodegeneration, and perhaps other CNS neurodegenerative diseases as well.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Role of hypoxia-inducible factor-1α in preconditioning-induced protection of retinal ganglion cells in glaucoma.Mol Vis. 2013 Nov 23;19:2360-72. eCollection 2013. Mol Vis. 2013. PMID: 24319330 Free PMC article.
-
Neuron stress and loss following rodent anterior ischemic optic neuropathy in double-reporter transgenic mice.Invest Ophthalmol Vis Sci. 2007 May;48(5):2304-10. doi: 10.1167/iovs.06-0486. Invest Ophthalmol Vis Sci. 2007. PMID: 17460295
-
Severe, early axonal degeneration following experimental anterior ischemic optic neuropathy.Invest Ophthalmol Vis Sci. 2014 Sep 23;55(11):7111-8. doi: 10.1167/iovs.14-14603. Invest Ophthalmol Vis Sci. 2014. PMID: 25249599
-
Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: Axon transport, injury and soma loss.Exp Eye Res. 2015 Dec;141:111-24. doi: 10.1016/j.exer.2015.06.006. Epub 2015 Jun 9. Exp Eye Res. 2015. PMID: 26070986 Review.
-
Autophagy in axonal degeneration in glaucomatous optic neuropathy.Prog Retin Eye Res. 2015 Jul;47:1-18. doi: 10.1016/j.preteyeres.2015.03.002. Epub 2015 Mar 26. Prog Retin Eye Res. 2015. PMID: 25816798 Review.
Cited by
-
Optimizing retinal ganglion cell nuclear staining for automated cell counting.Exp Eye Res. 2024 May;242:109881. doi: 10.1016/j.exer.2024.109881. Epub 2024 Mar 28. Exp Eye Res. 2024. PMID: 38554800
-
Intravitreal MPTP drives retinal ganglion cell loss with oral nicotinamide treatment providing robust neuroprotection.Acta Neuropathol Commun. 2024 May 21;12(1):79. doi: 10.1186/s40478-024-01782-3. Acta Neuropathol Commun. 2024. PMID: 38773545 Free PMC article.
-
Enhanced Retinal Ganglion Cell Survival in Glaucoma by Hypoxic Postconditioning After Disease Onset.Neurotherapeutics. 2015 Apr;12(2):502-14. doi: 10.1007/s13311-014-0330-x. Neurotherapeutics. 2015. PMID: 25549850 Free PMC article.
-
Role of C/EBP homologous protein in retinal ganglion cell death after ischemia/reperfusion injury.Invest Ophthalmol Vis Sci. 2014 Nov 20;56(1):221-31. doi: 10.1167/iovs.14-15447. Invest Ophthalmol Vis Sci. 2014. PMID: 25414185 Free PMC article.
-
A Novel NAD Signaling Mechanism in Axon Degeneration and its Relationship to Innate Immunity.Front Mol Biosci. 2021 Jul 8;8:703532. doi: 10.3389/fmolb.2021.703532. eCollection 2021. Front Mol Biosci. 2021. PMID: 34307460 Free PMC article. Review.
References
-
- Nickells RW, Semaan SJ, Schlamp CL. Involvement of the Bcl2 gene family in the signaling and control of retinal ganglion cell death. Prog Brain Res. 2008; 173: 423–435 - PubMed
-
- Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012; 31: 152–181 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

