Self-assembled antibody multimers through peptide nucleic acid conjugation
- PMID: 23210862
- PMCID: PMC3951380
- DOI: 10.1021/ja309505c
Self-assembled antibody multimers through peptide nucleic acid conjugation
Abstract
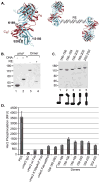
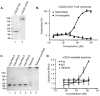
With the recent clinical success of bispecific antibodies, a strategy to rapidly synthesize and evaluate bispecific or higher order multispecific molecules could facilitate the discovery of new therapeutic agents. Here, we show that unnatural amino acids (UAAs) with orthogonal chemical reactivity can be used to generate site-specific antibody-oligonucleotide conjugates. These constructs can then be self-assembled into multimeric complexes with defined composition, valency, and geometry. With this approach, we generated potent bispecific antibodies that recruit cytotoxic T lymphocytes to Her2 and CD20 positive cancer cells, as well as multimeric antibody fragments with enhanced activity. This strategy should accelerate the synthesis and in vitro characterization of antibody constructs with unique specificities and molecular architectures.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
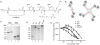

Similar articles
-
The eIg technology to generate Ig-like bispecific antibodies.MAbs. 2022 Jan-Dec;14(1):2063043. doi: 10.1080/19420862.2022.2063043. MAbs. 2022. PMID: 35427197 Free PMC article.
-
Site-specific coupling and sterically controlled formation of multimeric antibody fab fragments with unnatural amino acids.J Mol Biol. 2011 Mar 4;406(4):595-603. doi: 10.1016/j.jmb.2011.01.011. Epub 2011 Jan 13. J Mol Biol. 2011. PMID: 21237172 Free PMC article.
-
PSMA-targeted bispecific Fab conjugates that engage T cells.Bioorg Med Chem Lett. 2017 Dec 15;27(24):5490-5495. doi: 10.1016/j.bmcl.2017.09.065. Epub 2017 Oct 6. Bioorg Med Chem Lett. 2017. PMID: 29126850
-
Unnatural amino acids in novel antibody conjugates.Future Med Chem. 2014 Jul;6(11):1309-24. doi: 10.4155/fmc.14.79. Future Med Chem. 2014. PMID: 25163001 Review.
-
Antibody conjugates with unnatural amino acids.Mol Pharm. 2015 Jun 1;12(6):1848-62. doi: 10.1021/acs.molpharmaceut.5b00082. Epub 2015 May 21. Mol Pharm. 2015. PMID: 25898256 Review.
Cited by
-
Protein conjugation with genetically encoded unnatural amino acids.Curr Opin Chem Biol. 2013 Jun;17(3):412-9. doi: 10.1016/j.cbpa.2013.04.017. Epub 2013 May 9. Curr Opin Chem Biol. 2013. PMID: 23664497 Free PMC article. Review.
-
Modular Nucleic Acid Scaffolds for Synthesizing Monodisperse and Sequence-Encoded Antibody Oligomers.Chem. 2022 Nov 10;8(11):3018-3030. doi: 10.1016/j.chempr.2022.07.003. Epub 2022 Aug 5. Chem. 2022. PMID: 36405374 Free PMC article.
-
Rapid Systematic Screening of Bispecific Antibody Surrogate Geometries for T-Cell Engagement Using DNA Nanotechnology.J Am Chem Soc. 2024 Oct 30;146(43):29824-29835. doi: 10.1021/jacs.4c11648. Epub 2024 Oct 16. J Am Chem Soc. 2024. PMID: 39412838
-
A modular approach for assembling aldehyde-tagged proteins on DNA scaffolds.J Am Chem Soc. 2014 Aug 6;136(31):10850-3. doi: 10.1021/ja504711n. Epub 2014 Jul 25. J Am Chem Soc. 2014. PMID: 25029632 Free PMC article.
-
Expanding the scope of alkyne-mediated bioconjugations utilizing unnatural amino acids.Chem Commun (Camb). 2016 Jan 4;52(1):88-91. doi: 10.1039/c5cc08287k. Chem Commun (Camb). 2016. PMID: 26499242 Free PMC article.
References
-
- Holmes D. Nat Rev Drug Discov. 2011;10:798. - PubMed
-
- Carter P. Nat Rev Cancer. 2001;1:118. - PubMed
-
- Lum LG, Davol PA, Lee RJ. Exp Hematol. 2006;34:1. - PubMed
-
- Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandl C, Wolf A, Kirchinger P, Klappers P, Schmidt M, Riethmuller G, Reinhardt C, Baeuerle PA, Kufer P. Science. 2008;321:974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

