Salivary antigen SP32 is the immunodominant target of the antibody response to Phlebotomus papatasi bites in humans
- PMID: 23209854
- PMCID: PMC3510156
- DOI: 10.1371/journal.pntd.0001911
Salivary antigen SP32 is the immunodominant target of the antibody response to Phlebotomus papatasi bites in humans
Erratum in
-
Correction: Salivary Antigen SP32 Is the Immunodominant Target of the Antibody Response to Phlebotomus papatasi Bites in Humans.PLoS Negl Trop Dis. 2024 Jul 3;18(7):e0012303. doi: 10.1371/journal.pntd.0012303. eCollection 2024 Jul. PLoS Negl Trop Dis. 2024. PMID: 38959191 Free PMC article.
Abstract
Background: Zoonotic cutaneous leishmaniasis (ZCL) due to Leishmania major is highly prevalent in Tunisia and is transmitted by a hematophagous vector Phlebotomus papatasi (P. papatasi). While probing for a blood meal, the sand fly injects saliva into the host's skin, which contains a variety of compounds that are highly immunogenic. We recently showed that the presence of anti-saliva antibodies was associated with an enhanced risk for leishmaniasis and identified the immunodominant salivary protein of Phlebotomus papatasi as a protein of approximately 30 kDa.
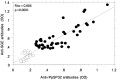
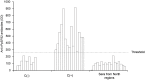
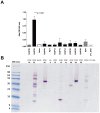
Methodology/principal findings: We cloned and expressed in mammalian cells two salivary proteins PpSP30 and PpSP32 with predicted molecular weights close to 30 kDa from the Tunisian strain of P. papatasi. The two recombinant salivary proteins were purified by two-step HPLC (High-Performance Liquid Chromatography) and tested if these proteins correspond to the immunodominant antigen of 30 kDa previously shown to be recognized by human sera from endemic areas for ZCL and exposed naturally to P. papatasi bites. While recombinant PpSP30 (rPpSP30) was poorly recognized by human sera from endemic areas for ZCL, rPpSP32 was strongly recognized by the tested sera. The binding of human IgG antibodies to native PpSP32 was inhibited by the addition of rPpSP32. Consistently, experiments in mice showed that PpSP32 induced the highest levels of antibodies compared to other P. papatasi salivary molecules while PpSP30 did not induce any detectable levels of antibodies.
Conclusions: Our findings demonstrate that PpSP32 is the immunodominant target of the antibody response to P. papatasi saliva. They also indicate that the recombinant form of PpSP32 is similar to the native one and represents a good candidate for large scale testing of human exposure to P. papatasi bites and perhaps for assessing the risk of contracting the disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Validation of Recombinant Salivary Protein PpSP32 as a Suitable Marker of Human Exposure to Phlebotomus papatasi, the Vector of Leishmania major in Tunisia.PLoS Negl Trop Dis. 2015 Sep 14;9(9):e0003991. doi: 10.1371/journal.pntd.0003991. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26368935 Free PMC article.
-
Kinetics of antibody response in BALB/c and C57BL/6 mice bitten by Phlebotomus papatasi.PLoS Negl Trop Dis. 2012;6(7):e1719. doi: 10.1371/journal.pntd.0001719. Epub 2012 Jul 10. PLoS Negl Trop Dis. 2012. Retraction in: PLoS Negl Trop Dis. 2023 Dec 22;17(12):e0011856. doi: 10.1371/journal.pntd.0011856 PMID: 22802977 Free PMC article. Retracted.
-
Human immune response to salivary proteins of wild-caught Phlebotomus papatasi.Parasitol Res. 2016 Sep;115(9):3345-55. doi: 10.1007/s00436-016-5094-2. Epub 2016 May 10. Parasitol Res. 2016. PMID: 27160331
-
Implicating bites from a leishmaniasis sand fly vector in the loss of tolerance in pemphigus.JCI Insight. 2020 Dec 3;5(23):e123861. doi: 10.1172/jci.insight.123861. JCI Insight. 2020. PMID: 33108348 Free PMC article.
-
Human antibody reaction against recombinant salivary proteins of Phlebotomus orientalis in Eastern Africa.PLoS Negl Trop Dis. 2018 Dec 4;12(12):e0006981. doi: 10.1371/journal.pntd.0006981. eCollection 2018 Dec. PLoS Negl Trop Dis. 2018. PMID: 30513081 Free PMC article.
Cited by
-
Canine Antibodies against Salivary Recombinant Proteins of Phlebotomus perniciosus: A Longitudinal Study in an Endemic Focus of Canine Leishmaniasis.PLoS Negl Trop Dis. 2015 Jun 25;9(6):e0003855. doi: 10.1371/journal.pntd.0003855. eCollection 2015. PLoS Negl Trop Dis. 2015. PMID: 26111018 Free PMC article.
-
Biomarkers for Zoonotic Visceral Leishmaniasis in Latin America.Front Cell Infect Microbiol. 2018 Jul 26;8:245. doi: 10.3389/fcimb.2018.00245. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30175073 Free PMC article. Review.
-
Recombinant Salivary Proteins of Phlebotomus orientalis are Suitable Antigens to Measure Exposure of Domestic Animals to Sand Fly Bites.PLoS Negl Trop Dis. 2016 Mar 17;10(3):e0004553. doi: 10.1371/journal.pntd.0004553. eCollection 2016 Mar. PLoS Negl Trop Dis. 2016. PMID: 26986566 Free PMC article.
-
Phlebotomus perniciosus Recombinant Salivary Proteins Polarize Murine Macrophages Toward the Anti-Inflammatory Phenotype.Front Cell Infect Microbiol. 2020 Aug 24;10:427. doi: 10.3389/fcimb.2020.00427. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32984064 Free PMC article.
-
Towards a Sustainable Vector-Control Strategy in the Post Kala-Azar Elimination Era.Front Cell Infect Microbiol. 2021 Mar 9;11:641632. doi: 10.3389/fcimb.2021.641632. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33768013 Free PMC article. Review.
References
-
- Aoun K, Amri F, Chouihi E, Haouas N, Bedoui K, et al. (2008) Epidemiology of Leishmania infantum, L. major and L. killicki in Tunisia: results and analysis of the identification of 226 human and canine isolates. Bull Soc Pathol Exot 101: 323–328. - PubMed
-
- Barral A, Honda E, Caldas A, Costa J, Vinhas V, et al. (2000) Human immune response to sand fly salivary gland antigens: a useful epidemiological marker? Am J Trop Med Hyg 62: 740–745. - PubMed
-
- Vinhas V, Andrade BB, Paes F, Bomura A, Clarencio J, et al. (2007) Human anti-saliva immune response following experimental exposure to the visceral leishmaniasis vector, Lutzomyia longipalpis . Eur J Immunol 37: 3111–3121. - PubMed
-
- Rohousova I, Ozensoy S, Ozbel Y, Volf P (2005) Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology 130: 493–499. - PubMed
-
- Silva F, Gomes R, Prates D, Miranda JC, Andrade B, et al. (2005) Inflammatory cell infiltration and high antibody production in BALB/c mice caused by natural exposure to Lutzomyia longipalpis bites. Am J Trop Med Hyg 72: 94–98. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

