Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling
- PMID: 23209312
- PMCID: PMC3526353
- DOI: 10.1084/jem.20120873
Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling
Abstract
The endogenous phospholipid lysophosphatidic acid (LPA) regulates fundamental cellular processes such as proliferation, survival, motility, and invasion implicated in homeostatic and pathological conditions. Hence, delineation of the full range of molecular mechanisms by which LPA exerts its broad effects is essential. We report avid binding of LPA to the receptor for advanced glycation end products (RAGE), a member of the immunoglobulin superfamily, and mapping of the LPA binding site on this receptor. In vitro, RAGE was required for LPA-mediated signal transduction in vascular smooth muscle cells and C6 glioma cells, as well as proliferation and migration. In vivo, the administration of soluble RAGE or genetic deletion of RAGE mitigated LPA-stimulated vascular Akt signaling, autotaxin/LPA-driven phosphorylation of Akt and cyclin D1 in the mammary tissue of transgenic mice vulnerable to carcinogenesis, and ovarian tumor implantation and development. These findings identify novel roles for RAGE as a conduit for LPA signaling and suggest targeting LPA-RAGE interaction as a therapeutic strategy to modify the pathological actions of LPA.
Figures
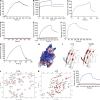

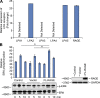
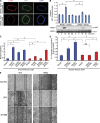
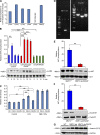
Similar articles
-
Amelioration of human peritoneal mesothelial cell co-culture-evoked malignant potential of ovarian cancer cells by acacetin involves LPA release-activated RAGE-PI3K/AKT signaling.Cell Mol Biol Lett. 2021 Dec 9;26(1):51. doi: 10.1186/s11658-021-00296-3. Cell Mol Biol Lett. 2021. PMID: 34886812 Free PMC article.
-
Lysophosphatidic acid-RAGE axis promotes lung and mammary oncogenesis via protein kinase B and regulating tumor microenvironment.Cell Commun Signal. 2020 Oct 27;18(1):170. doi: 10.1186/s12964-020-00666-y. Cell Commun Signal. 2020. PMID: 33109194 Free PMC article.
-
Signaling of Serum Amyloid A Through Receptor for Advanced Glycation End Products as a Possible Mechanism for Uremia-Related Atherosclerosis.Arterioscler Thromb Vasc Biol. 2016 May;36(5):800-9. doi: 10.1161/ATVBAHA.115.306349. Epub 2016 Mar 17. Arterioscler Thromb Vasc Biol. 2016. PMID: 26988587
-
Roles for lysophosphatidic acid signaling in vascular development and disease.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Aug;1865(8):158734. doi: 10.1016/j.bbalip.2020.158734. Epub 2020 May 3. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 32376340 Review.
-
Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis.Circ Res. 1999 Mar 19;84(5):489-97. doi: 10.1161/01.res.84.5.489. Circ Res. 1999. PMID: 10082470 Review.
Cited by
-
Emerging Role of Phospholipase-Derived Cleavage Products in Regulating Eosinophil Activity: Focus on Lysophospholipids, Polyunsaturated Fatty Acids and Eicosanoids.Int J Mol Sci. 2021 Apr 21;22(9):4356. doi: 10.3390/ijms22094356. Int J Mol Sci. 2021. PMID: 33919453 Free PMC article. Review.
-
The RAGE/DIAPH1 Signaling Axis & Implications for the Pathogenesis of Diabetic Complications.Int J Mol Sci. 2022 Apr 21;23(9):4579. doi: 10.3390/ijms23094579. Int J Mol Sci. 2022. PMID: 35562970 Free PMC article. Review.
-
Glycation and a Spark of ALEs (Advanced Lipoxidation End Products) - Igniting RAGE/Diaphanous-1 and Cardiometabolic Disease.Front Cardiovasc Med. 2022 Jun 24;9:937071. doi: 10.3389/fcvm.2022.937071. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35811725 Free PMC article. Review.
-
Amelioration of human peritoneal mesothelial cell co-culture-evoked malignant potential of ovarian cancer cells by acacetin involves LPA release-activated RAGE-PI3K/AKT signaling.Cell Mol Biol Lett. 2021 Dec 9;26(1):51. doi: 10.1186/s11658-021-00296-3. Cell Mol Biol Lett. 2021. PMID: 34886812 Free PMC article.
-
Unlocking the biology of RAGE in diabetic microvascular complications.Trends Endocrinol Metab. 2014 Jan;25(1):15-22. doi: 10.1016/j.tem.2013.08.002. Epub 2013 Sep 3. Trends Endocrinol Metab. 2014. PMID: 24011512 Free PMC article. Review.
References
-
- Bu D.X., Rai V., Shen X., Rosario R., Lu Y., D’Agati V., Yan S.F., Friedman R.A., Nuglozeh E., Schmidt A.M. 2010. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ. Res. 106:1040–1051 10.1161/CIRCRESAHA.109.201103 - DOI - PMC - PubMed
-
- Chen Y., Yan S.S., Colgan J., Zhang H.P., Luban J., Schmidt A.M., Stern D., Herold K.C. 2004. Blockade of late stages of autoimmune diabetes by inhibition of the receptor for advanced glycation end products. J. Immunol. 173:1399–1405 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

