Human ferredoxin-2 displays a unique conformational change
- PMID: 23208207
- PMCID: PMC3622203
- DOI: 10.1039/c2dt32018e
Human ferredoxin-2 displays a unique conformational change
Abstract
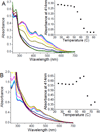
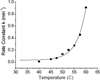
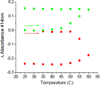
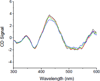
Human ferredoxin-1 (hFd1) and human ferredoxin-2 (hFd2) share high sequence similarity but serve on distinct cellular pathways. A unique conformational change is observed when holo hFd2 is warmed to physiological temperatures, or higher. Enzymatic studies show that this conformational change causes the increase of affinity between hFd2 and adrenodoxin reductase. No such change was observed for hFd1, which may contribute to the distinct cellular functions of hFd1 and hFd2 under physiological conditions.
Figures
Similar articles
-
Humans possess two mitochondrial ferredoxins, Fdx1 and Fdx2, with distinct roles in steroidogenesis, heme, and Fe/S cluster biosynthesis.Proc Natl Acad Sci U S A. 2010 Jun 29;107(26):11775-80. doi: 10.1073/pnas.1004250107. Epub 2010 Jun 14. Proc Natl Acad Sci U S A. 2010. PMID: 20547883 Free PMC article.
-
Energetic basis on interactions between ferredoxin and ferredoxin NADP+ reductase at varying physiological conditions.Biochem Biophys Res Commun. 2017 Jan 22;482(4):909-915. doi: 10.1016/j.bbrc.2016.11.132. Epub 2016 Nov 25. Biochem Biophys Res Commun. 2017. PMID: 27894842
-
On the specificity of pig adrenal ferredoxin (adrenodoxin) and spinach ferredoxin in electron-transfer reactions.Eur J Biochem. 1988 Jul 1;174(4):629-35. doi: 10.1111/j.1432-1033.1988.tb14144.x. Eur J Biochem. 1988. PMID: 2839337
-
Adrenodoxin: the archetype of vertebrate-type [2Fe-2S] cluster ferredoxins.Biochim Biophys Acta. 2011 Jan;1814(1):111-25. doi: 10.1016/j.bbapap.2010.06.003. Epub 2010 Jun 9. Biochim Biophys Acta. 2011. PMID: 20538075 Review.
-
Adrenodoxin: structure, stability, and electron transfer properties.Proteins. 2000 Sep 1;40(4):590-612. doi: 10.1002/1097-0134(20000901)40:4<590::aid-prot50>3.0.co;2-p. Proteins. 2000. PMID: 10899784 Review.
Cited by
-
Reconstitution, characterization, and [2Fe-2S] cluster exchange reactivity of a holo human BOLA3 homodimer.J Biol Inorg Chem. 2019 Oct;24(7):1035-1045. doi: 10.1007/s00775-019-01713-x. Epub 2019 Sep 5. J Biol Inorg Chem. 2019. PMID: 31486956 Free PMC article.
-
Cluster exchange reactivity of [2Fe-2S] cluster-bridged complexes of BOLA3 with monothiol glutaredoxins.Metallomics. 2018 Sep 19;10(9):1282-1290. doi: 10.1039/c8mt00128f. Metallomics. 2018. PMID: 30137089 Free PMC article.
-
Cluster and fold stability of E. coli ISC-type ferredoxin.PLoS One. 2013 Nov 12;8(11):e78948. doi: 10.1371/journal.pone.0078948. eCollection 2013. PLoS One. 2013. PMID: 24265733 Free PMC article.
-
Regulation of human Nfu activity in Fe-S cluster delivery-characterization of the interaction between Nfu and the HSPA9/Hsc20 chaperone complex.FEBS J. 2018 Jan;285(2):391-410. doi: 10.1111/febs.14353. Epub 2017 Dec 29. FEBS J. 2018. PMID: 29211945 Free PMC article.
-
Mapping cellular Fe-S cluster uptake and exchange reactions - divergent pathways for iron-sulfur cluster delivery to human ferredoxins.Metallomics. 2016 Dec 7;8(12):1283-1293. doi: 10.1039/c6mt00193a. Metallomics. 2016. PMID: 27878189 Free PMC article.
References
-
- Vickery LE. Steroids. 1997;62:124–127. - PubMed
-
- Grinberg AV, Hannemann F, Schiffler B, Muller J, Heinemann U, Bernhardt R. Proteins. 2000;40:590–612. - PubMed
-
- Miller WL. Endocrinology. 2005;146:2544–2550. - PubMed
-
- Lill R. Nature. 2009;460:831–838. - PubMed
-
- Johnson DC, Dean DR, Smith AD, Johnson MK. Ann. Rev Biochem. 2005;74:247–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

