Advanced vaccine candidates for Lassa fever
- PMID: 23202493
- PMCID: PMC3509661
- DOI: 10.3390/v4112514
Advanced vaccine candidates for Lassa fever
Abstract
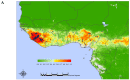
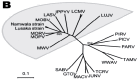
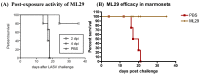
Lassa virus (LASV) is the most prominent human pathogen of the Arenaviridae. The virus is transmitted to humans by a rodent reservoir, Mastomys natalensis, and is capable of causing lethal Lassa Fever (LF). LASV has the highest human impact of any of the viral hemorrhagic fevers (with the exception of Dengue Fever) with an estimated several hundred thousand infections annually, resulting in thousands of deaths in Western Africa. The sizeable disease burden, numerous imported cases of LF in non-endemic countries, and the possibility that LASV can be used as an agent of biological warfare make a strong case for vaccine development. Presently there is no licensed vaccine against LF or approved treatment. Recently, several promising vaccine candidates have been developed which can potentially target different groups at risk. The purpose of this manuscript is to review the LASV pathogenesis and immune mechanisms involved in protection. The current status of pre-clinical development of the advanced vaccine candidates that have been tested in non-human primates will be discussed. Major scientific, manufacturing, and regulatory challenges will also be considered.
Figures


Similar articles
-
The search for animal models for Lassa fever vaccine development.Expert Rev Vaccines. 2013 Jan;12(1):71-86. doi: 10.1586/erv.12.139. Expert Rev Vaccines. 2013. PMID: 23256740 Free PMC article. Review.
-
A Lassa Fever Live-Attenuated Vaccine Based on Codon Deoptimization of the Viral Glycoprotein Gene.mBio. 2020 Feb 25;11(1):e00039-20. doi: 10.1128/mBio.00039-20. mBio. 2020. PMID: 32098811 Free PMC article.
-
Vaccine platforms to control Lassa fever.Expert Rev Vaccines. 2016 Sep;15(9):1135-50. doi: 10.1080/14760584.2016.1184575. Epub 2016 May 24. Expert Rev Vaccines. 2016. PMID: 27136941 Review.
-
Vaccine platforms for the prevention of Lassa fever.Immunol Lett. 2019 Nov;215:1-11. doi: 10.1016/j.imlet.2019.03.008. Epub 2019 Apr 23. Immunol Lett. 2019. PMID: 31026485 Free PMC article. Review.
-
Current research for a vaccine against Lassa hemorrhagic fever virus.Drug Des Devel Ther. 2018 Aug 14;12:2519-2527. doi: 10.2147/DDDT.S147276. eCollection 2018. Drug Des Devel Ther. 2018. PMID: 30147299 Free PMC article. Review.
Cited by
-
Development of live-attenuated arenavirus vaccines based on codon deoptimization.J Virol. 2015 Apr;89(7):3523-33. doi: 10.1128/JVI.03401-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589652 Free PMC article.
-
Baseline mapping of Lassa fever virology, epidemiology and vaccine research and development.NPJ Vaccines. 2018 Mar 20;3:11. doi: 10.1038/s41541-018-0049-5. eCollection 2018. NPJ Vaccines. 2018. PMID: 29581897 Free PMC article. Review.
-
Lassa Virus, but Not Highly Pathogenic New World Arenaviruses, Restricts Immunostimulatory Double-Stranded RNA Accumulation during Infection.J Virol. 2020 Apr 16;94(9):e02006-19. doi: 10.1128/JVI.02006-19. Print 2020 Apr 16. J Virol. 2020. PMID: 32051278 Free PMC article.
-
Enhanced Efficacy of a Codon-Optimized DNA Vaccine Encoding the Glycoprotein Precursor Gene of Lassa Virus in a Guinea Pig Disease Model When Delivered by Dermal Electroporation.Vaccines (Basel). 2013 Jul 18;1(3):262-77. doi: 10.3390/vaccines1030262. Vaccines (Basel). 2013. PMID: 26344112 Free PMC article.
-
An attenuated Lassa vaccine in SIV-infected rhesus macaques does not persist or cause arenavirus disease but does elicit Lassa virus-specific immunity.Virol J. 2013 Feb 12;10:52. doi: 10.1186/1743-422X-10-52. Virol J. 2013. PMID: 23402317 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

