1α,25-dihydroxyvitamin D3 modulates the hair-inductive capacity of dermal papilla cells: therapeutic potential for hair regeneration
- PMID: 23197867
- PMCID: PMC3659730
- DOI: 10.5966/sctm.2012-0032
1α,25-dihydroxyvitamin D3 modulates the hair-inductive capacity of dermal papilla cells: therapeutic potential for hair regeneration
Abstract
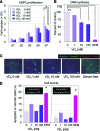
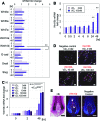
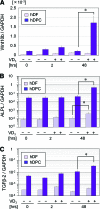
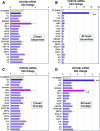
Dermal papilla cells (DPCs) have the potential to induce differentiation of epithelial stem cells into hair, and Wnt signaling is deeply involved in the initiation process. The functional limitation of expanded adult DPCs has been a difficult challenge for cell-based hair regrowth therapy. We previously reported that 1α,25-dihydroxyvitamin D(3) (VD(3)) upregulates expression of transforming growth factor (TGF)-β2 and alkaline phosphatase (ALP) activity, both features of hair-inducing human DPCs (hDPCs). In this study, we further examined the effects and signaling pathways associated with VD(3) actions on DPCs. VD(3) suppressed hDPC proliferation in a dose-dependent, noncytotoxic manner. Among the Wnt-related genes investigated, Wnt10b expression was significantly upregulated by VD(3) in hDPCs. Wnt10b upregulation, as well as upregulation of ALPL (ALP, liver/bone/kidney) and TGF-β2, by VD(3) was specific in hDPCs and not detected in human dermal fibroblasts. Screening of paracrine or endocrine factors in the skin indicated that all-trans retinoic acid (atRA) upregulated Wnt10b gene expression, although synergistic upregulation (combined atRA and VD(3)) was not seen. RNA interference with vitamin D receptor (VDR) revealed that VD(3) upregulation of Wnt10b, ALPL, and TGF-β2 was mediated through the genomic VDR pathway. In a rat model of de novo hair regeneration by murine DPC transplantation, pretreatment with VD(3) significantly enhanced hair folliculogenesis. Specifically, a greater number of outgrowing hair shafts and higher maturation of regenerated follicles were observed. Together, these data suggest that VD(3) may promote functional differentiation of DPCs and be useful in preserving the hair follicle-inductive capacity of cultured DPCs for hair regeneration therapies.
Figures


Similar articles
-
TGF-beta is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis.J Cell Mol Med. 2009 Nov-Dec;13(11-12):4643-56. doi: 10.1111/j.1582-4934.2009.00739.x. Epub 2009 Mar 6. J Cell Mol Med. 2009. PMID: 19438810 Free PMC article.
-
Wnt10b promotes hair follicles growth and dermal papilla cells proliferation via Wnt/β-Catenin signaling pathway in Rex rabbits.Biosci Rep. 2020 Feb 28;40(2):BSR20191248. doi: 10.1042/BSR20191248. Biosci Rep. 2020. PMID: 31961392 Free PMC article.
-
Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of transforming growth factor-beta2 in the dermal papilla.J Invest Dermatol. 2005 Jun;124(6):1119-26. doi: 10.1111/j.0022-202X.2005.23686.x. J Invest Dermatol. 2005. PMID: 15955085
-
Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods.Skin Pharmacol Physiol. 2020;33(5):280-292. doi: 10.1159/000510152. Epub 2020 Oct 14. Skin Pharmacol Physiol. 2020. PMID: 33053562 Review.
-
Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth.Int J Cosmet Sci. 2018 Oct;40(5):429-450. doi: 10.1111/ics.12489. Epub 2018 Oct 4. Int J Cosmet Sci. 2018. PMID: 30144361 Review.
Cited by
-
Nature-derived lignan compound VB-1 exerts hair growth-promoting effects by augmenting Wnt/β-catenin signaling in human dermal papilla cells.PeerJ. 2018 May 8;6:e4737. doi: 10.7717/peerj.4737. eCollection 2018. PeerJ. 2018. PMID: 29761053 Free PMC article.
-
Chemotherapy-induced alopecia management: Clinical experience and practical advice.J Cosmet Dermatol. 2017 Dec;16(4):537-541. doi: 10.1111/jocd.12308. Epub 2017 Feb 2. J Cosmet Dermatol. 2017. PMID: 28150447 Free PMC article. Review.
-
A systematic summary of survival and death signalling during the life of hair follicle stem cells.Stem Cell Res Ther. 2021 Aug 11;12(1):453. doi: 10.1186/s13287-021-02527-y. Stem Cell Res Ther. 2021. PMID: 34380571 Free PMC article. Review.
-
Influence of Nutrition, Food Supplements and Lifestyle in Hair Disorders.Indian Dermatol Online J. 2022 Oct 21;13(6):721-724. doi: 10.4103/idoj.idoj_175_22. eCollection 2022 Nov-Dec. Indian Dermatol Online J. 2022. PMID: 36386748 Free PMC article. Review.
-
CRISPR/Cas9-mediated VDR knockout plays an essential role in the growth of dermal papilla cells through enhanced relative genes.PeerJ. 2019 Jul 3;7:e7230. doi: 10.7717/peerj.7230. eCollection 2019. PeerJ. 2019. PMID: 31309000 Free PMC article.
References
-
- Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: Vibrissa-type fibres are specified. Development. 1992;115:1103–1109. - PubMed
-
- Jahoda CA, Reynolds AJ, Oliver RF. Induction of hair growth in ear wounds by cultured dermal papilla cells. J Invest Dermatol. 1993;101:584–590. - PubMed
-
- Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992;115:587–593. - PubMed
-
- Lichti U, Weinberg WC, Goodman L, et al. In vivo regulation of murine hair growth: Insights from grafting defined cell populations onto nude mice. J Invest Dermatol. 1993;101:124S–129S. - PubMed
-
- Weinberg WC, Goodman LV, George C, et al. Reconstitution of hair follicle development in vivo: Determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol. 1993;100:229–236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

