Cooperating transcription factors mediate the function of estrogen receptor
- PMID: 23192763
- PMCID: PMC3608891
- DOI: 10.1007/s00412-012-0392-7
Cooperating transcription factors mediate the function of estrogen receptor
Abstract
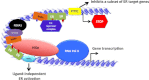
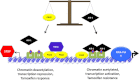
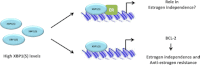
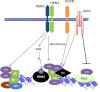
Estrogen receptor (ER) is a hormone-regulated transcription factor that controls cell division and differentiation in the ovary, breast, and uterus. The expression of ER is a common feature of the majority of breast cancers, which is used as a therapeutic target. Recent genetic studies have shown that ER binding occurs in regions distant to the promoters of estrogen target genes. These studies have also demonstrated that ER binding is accompanied with the binding of other transcription factors, which regulate the function of ER and response to anti-estrogen therapies. In this review, we explain how these factors influence the interaction of ER to chromatin and their cooperation for ER transcriptional activity. Moreover, we describe how the expression of these factors dictates the response to anti-estrogen therapies. Finally, we discuss how cytoplasmatic signaling pathways may modulate the function of ER and its cooperating transcription factors.
Figures

Similar articles
-
Association of FGFR1 with ERα Maintains Ligand-Independent ER Transcription and Mediates Resistance to Estrogen Deprivation in ER+ Breast Cancer.Clin Cancer Res. 2017 Oct 15;23(20):6138-6150. doi: 10.1158/1078-0432.CCR-17-1232. Epub 2017 Jul 27. Clin Cancer Res. 2017. PMID: 28751448 Free PMC article.
-
Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes.Cancer Res. 2006 Jun 15;66(12):6370-8. doi: 10.1158/0008-5472.CAN-06-0402. Cancer Res. 2006. PMID: 16778215 Free PMC article.
-
Twist contributes to hormone resistance in breast cancer by downregulating estrogen receptor-α.Oncogene. 2012 Jul 5;31(27):3223-34. doi: 10.1038/onc.2011.483. Epub 2011 Nov 7. Oncogene. 2012. PMID: 22056872 Free PMC article.
-
Estrogen signaling and unfolded protein response in breast cancer.J Steroid Biochem Mol Biol. 2016 Oct;163:45-50. doi: 10.1016/j.jsbmb.2016.03.036. Epub 2016 Apr 1. J Steroid Biochem Mol Biol. 2016. PMID: 27045680 Review.
-
Estrogen receptor-positive (ER+) breast cancer treatment: Are multi-target compounds the next promising approach?Biochem Pharmacol. 2020 Jul;177:113989. doi: 10.1016/j.bcp.2020.113989. Epub 2020 Apr 21. Biochem Pharmacol. 2020. PMID: 32330493 Review.
Cited by
-
Identification of potential new treatment response markers and therapeutic targets using a Gaussian process-based method in lapatinib insensitive breast cancer models.PLoS One. 2017 May 8;12(5):e0177058. doi: 10.1371/journal.pone.0177058. eCollection 2017. PLoS One. 2017. PMID: 28481952 Free PMC article.
-
Emerging role of pioneer transcription factors in targeted ERα positive breast cancer.Explor Target Antitumor Ther. 2021;2(1):26-35. doi: 10.37349/etat.2021.00031. Epub 2021 Feb 28. Explor Target Antitumor Ther. 2021. PMID: 36046086 Free PMC article. Review.
-
Molecular characterization of Wdr13 knockout female mice uteri: a model for human endometrial hyperplasia.Sci Rep. 2020 Sep 3;10(1):14621. doi: 10.1038/s41598-020-70773-w. Sci Rep. 2020. PMID: 32883989 Free PMC article.
-
Genetic and epigenetic factors affect RET gene expression in breast cancer cell lines and influence survival in patients.Oncotarget. 2016 May 3;7(18):26465-79. doi: 10.18632/oncotarget.8417. Oncotarget. 2016. PMID: 27034161 Free PMC article.
-
Expression analysis of the estrogen receptor target genes in renal cell carcinoma.Mol Med Rep. 2015 Jan;11(1):75-82. doi: 10.3892/mmr.2014.2766. Epub 2014 Oct 24. Mol Med Rep. 2015. PMID: 25351113 Free PMC article.
References
-
- Ademuyiwa FO, Thorat MA, et al. Expression of forkhead-box protein A1, a marker of luminal A type breast cancer, parallels low oncotype DX 21-gene recurrence scores. Mod Pathol. 2010;23(2):270–275. - PubMed
-
- Anzick SL, Kononen J, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277(5328):965–968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

