Acquisition of paclitaxel resistance is associated with a more aggressive and invasive phenotype in prostate cancer
- PMID: 23192682
- PMCID: PMC4211414
- DOI: 10.1002/jcb.24464
Acquisition of paclitaxel resistance is associated with a more aggressive and invasive phenotype in prostate cancer
Abstract
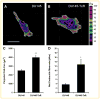
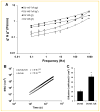
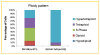
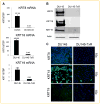
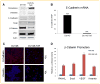
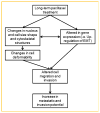
Drug resistance is a major limitation to the successful treatment of advanced prostate cancer (PCa). Patients who have metastatic, castration-resistant PCa (mCRPC) are treated with chemotherapeutics. However, these standard therapy modalities culminate in the development of resistance. We established paclitaxel resistance in a classic, androgen-insensitive mCRPC cell line (DU145) and, using a suite of molecular and biophysical methods, characterized the structural and functional changes in vitro and in vivo that are associated with the development of drug resistance. After acquiring paclitaxel-resistance, cells exhibited an abnormal nuclear morphology with extensive chromosomal content, an increase in stiffness, and faster cytoskeletal remodeling dynamics. Compared with the parental DU145, paclitaxel-resistant (DU145-TxR) cells became highly invasive and motile in vitro, exercised greater cell traction forces, and formed larger and rapidly growing tumors in mouse xenografts. Furthermore, DU145-TxR cells showed a discrete loss of keratins but a distinct gain of ZEB1, Vimentin and Snail, suggesting an epithelial-to-mesenchymal transition. These findings demonstrate, for the first time, that paclitaxel resistance in PCa is associated with a trans-differentiation of epithelial cell machinery that enables more aggressive and invasive phenotype and portend new strategies for developing novel biomarkers and effective treatment modalities for PCa patients.
Copyright © 2012 Wiley Periodicals, Inc.
Figures

Similar articles
-
Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling.Chin J Cancer. 2017 Mar 29;36(1):35. doi: 10.1186/s40880-017-0203-x. Chin J Cancer. 2017. PMID: 28356132 Free PMC article.
-
Downregulation of cytokeratin 18 is associated with paclitaxel‑resistance and tumor aggressiveness in prostate cancer.Int J Oncol. 2016 Apr;48(4):1730-6. doi: 10.3892/ijo.2016.3396. Epub 2016 Feb 17. Int J Oncol. 2016. PMID: 26892177
-
Knockdown of lncRNA CCAT1 enhances sensitivity of paclitaxel in prostate cancer via regulating miR-24-3p and FSCN1.Cancer Biol Ther. 2020 May 3;21(5):452-462. doi: 10.1080/15384047.2020.1727700. Epub 2020 Feb 23. Cancer Biol Ther. 2020. PMID: 32089062 Free PMC article.
-
The establishment of two paclitaxel-resistant prostate cancer cell lines and the mechanisms of paclitaxel resistance with two cell lines.Prostate. 2007 Jun 15;67(9):955-67. doi: 10.1002/pros.20581. Prostate. 2007. PMID: 17440963
-
Apoptosis evasion: the role of survival pathways in prostate cancer progression and therapeutic resistance.J Cell Biochem. 2006 Jan 1;97(1):18-32. doi: 10.1002/jcb.20634. J Cell Biochem. 2006. PMID: 16216007 Free PMC article. Review.
Cited by
-
Phenotypic Basis for Matrix Stiffness-Dependent Chemoresistance of Breast Cancer Cells to Doxorubicin.Front Oncol. 2018 Sep 5;8:337. doi: 10.3389/fonc.2018.00337. eCollection 2018. Front Oncol. 2018. PMID: 30234012 Free PMC article.
-
Morin promotes prostate cancer cells chemosensitivity to paclitaxel through miR-155/GATA3 axis.Oncotarget. 2017 Jul 18;8(29):47849-47860. doi: 10.18632/oncotarget.18133. Oncotarget. 2017. PMID: 28599307 Free PMC article.
-
Combinatorial TGF-β attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells.Oncotarget. 2015 Nov 10;6(35):37526-43. doi: 10.18632/oncotarget.6063. Oncotarget. 2015. PMID: 26462028 Free PMC article.
-
Chronic arsenic trioxide exposure leads to enhanced aggressiveness via Met oncogene addiction in cancer cells.Oncotarget. 2016 May 10;7(19):27379-93. doi: 10.18632/oncotarget.8415. Oncotarget. 2016. PMID: 27036042 Free PMC article.
-
A Tale of Two States: Normal and Transformed, With and Without Rigidity Sensing.Annu Rev Cell Dev Biol. 2019 Oct 6;35:169-190. doi: 10.1146/annurev-cellbio-100818-125227. Epub 2019 Aug 14. Annu Rev Cell Dev Biol. 2019. PMID: 31412209 Free PMC article. Review.
References
-
- Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A’Hern R, Levink R, Coumans F, Moreira J, Riisnaes R, Oommen NB, Hawche G, Jameson C, Thompson E, Sipkema R, Carden CP, Parker C, Dearnaley D, Kaye SB, Cooper CS, Molina A, Cox ME, Terstappen LW, de Bono JS. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69:2912–2918. - PubMed
-
- Bracarda S, Logothetis C, Sternberg CN, Oudard S. Current and emerging treatment modalities for metastatic castration-resistant prostate cancer. BJU Int. 2011;107(Suppl 2):13–20. - PubMed
-
- Bursac P, Lenormand G, Fabry B, Oliver M, Weitz DA, Viasnoff V, Butler JP, Fredberg JJ. Cytoskeletal remodelling and slow dynamics in the living cell. Nat Mater. 2005;4:557–561. - PubMed
-
- Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21:2679–2694. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

