Lentiviral vector gene transfer to porcine airways
- PMID: 23187455
- PMCID: PMC3511674
- DOI: 10.1038/mtna.2012.47
Lentiviral vector gene transfer to porcine airways
Abstract
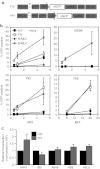
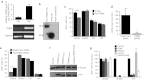
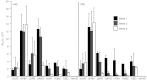
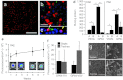
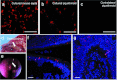
In this study, we investigated lentiviral vector development and transduction efficiencies in well-differentiated primary cultures of pig airway epithelia (PAE) and wild-type pigs in vivo. We noted gene transfer efficiencies similar to that observed for human airway epithelia (HAE). Interestingly, feline immunodeficiency virus (FIV)-based vectors transduced immortalized pig cells as well as pig primary cells more efficiently than HIV-1-based vectors. PAE express TRIM5α, a well-characterized species-specific lentiviral restriction factor. We contrasted the restrictive properties of porcine TRIM5α against FIV- and HIV-based vectors using gain and loss of function approaches. We observed no effect on HIV-1 or FIV conferred transgene expression in response to porcine TRIM5α overexpression or knockdown. To evaluate the ability of GP64-FIV to transduce porcine airways in vivo, we delivered vector expressing mCherry to the tracheal lobe of the lung and the ethmoid sinus of 4-week-old pigs. One week later, epithelial cells expressing mCherry were readily detected. Our findings indicate that pseudotyped FIV vectors confer similar tropisms in porcine epithelia as observed in human HAE and provide further support for the selection of GP64 as an appropriate envelope pseudotype for future preclinical gene therapy studies in the porcine model of cystic fibrosis (CF).Molecular Therapy - Nucleic Acids (2012) 1, e56; doi:10.1038/mtna.2012.47; published online 27 November 2012.
Figures

Similar articles
-
Intrapulmonary Versus Nasal Transduction of Murine Airways With GP64-pseudotyped Viral Vectors.Mol Ther Nucleic Acids. 2013 Jan 29;2(1):e69. doi: 10.1038/mtna.2012.60. Mol Ther Nucleic Acids. 2013. PMID: 23360952 Free PMC article.
-
Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus-based vector gene transfer.J Virol. 2005 Oct;79(20):12818-27. doi: 10.1128/JVI.79.20.12818-12827.2005. J Virol. 2005. PMID: 16188984 Free PMC article.
-
Human, Pig, and Mouse Interferon-Induced Transmembrane Proteins Partially Restrict Pseudotyped Lentiviral Vectors.Hum Gene Ther. 2016 May;27(5):354-62. doi: 10.1089/hum.2015.156. Hum Gene Ther. 2016. PMID: 27004832 Free PMC article.
-
Lentiviral Vectors for the Treatment and Prevention of Cystic Fibrosis Lung Disease.Genes (Basel). 2019 Mar 14;10(3):218. doi: 10.3390/genes10030218. Genes (Basel). 2019. PMID: 30875857 Free PMC article. Review.
-
FIV vector systems.Somat Cell Mol Genet. 2001 Nov;26(1-6):99-129. doi: 10.1023/a:1021078714105. Somat Cell Mol Genet. 2001. PMID: 12465464 Review.
Cited by
-
Progress in Respiratory Gene Therapy.Hum Gene Ther. 2022 Sep;33(17-18):893-912. doi: 10.1089/hum.2022.172. Hum Gene Ther. 2022. PMID: 36074947 Free PMC article. Review.
-
Cystic Fibrosis Gene Therapy: Looking Back, Looking Forward.Genes (Basel). 2018 Nov 7;9(11):538. doi: 10.3390/genes9110538. Genes (Basel). 2018. PMID: 30405068 Free PMC article. Review.
-
Gene Therapy Applications of Non-Human Lentiviral Vectors.Viruses. 2020 Sep 29;12(10):1106. doi: 10.3390/v12101106. Viruses. 2020. PMID: 33003635 Free PMC article. Review.
-
New insights into the pathogenesis of cystic fibrosis sinusitis.Int Forum Allergy Rhinol. 2014 Feb;4(2):132-7. doi: 10.1002/alr.21252. Epub 2013 Nov 26. Int Forum Allergy Rhinol. 2014. PMID: 24282147 Free PMC article. Review.
-
Advanced approaches to overcome biological barriers in respiratory and systemic routes of administration for enhanced nucleic acid delivery to the lung.Expert Opin Drug Deliv. 2023 Jul-Dec;20(11):1531-1552. doi: 10.1080/17425247.2023.2282535. Epub 2023 Dec 20. Expert Opin Drug Deliv. 2023. PMID: 37946533 Free PMC article. Review.
References
-
- Clarke LL., and, Boucher RC. Chloride secretory response to extracellular ATP in human normal and cystic fibrosis nasal epithelia. Am J Physiol. 1992;263 2 Pt 1:C348–C356. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

