GABAB receptor subtypes differentially modulate synaptic inhibition in the dentate gyrus to enhance granule cell output
- PMID: 23186302
- PMCID: PMC3623052
- DOI: 10.1111/bph.12073
GABAB receptor subtypes differentially modulate synaptic inhibition in the dentate gyrus to enhance granule cell output
Abstract
Background and purpose: Activation of GABAB receptors in the dentate gyrus (DG) enhances granule cell (GC) activity by reducing synaptic inhibition imposed by hilar interneurons. This disinhibitory action facilitates signal transfer from the perforant path to the hippocampus. However, as the two main molecular subtypes, GABA(B(1a,2)) and GABA(B(1b,2)) receptors, prefer axonal terminal and dendritic compartments, respectively, they may modulate the hilar pathways at different synaptic localizations. We examined their relative expression and functions in the DG.
Experimental approach: The localization of GABAB subtypes was revealed immunohistochemically using subunit-selective antibodies in GABA(B1a)(-/-) and GABA(B1b)(-/-) mice. Effects of subtype activation by the GABAB receptor agonist, baclofen, were examined on the perforant path-stimulated GC population activities in brain slices.
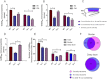
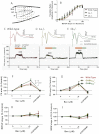
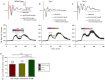
Key results: GABA(B(1a,2)) receptors were concentrated in the inner molecular layer, the neuropil of the hilus and hilar neurons at the border zone; while GABA(B(1b,2)) receptors dominated the outer molecular layer and hilar neurons in the deep layer, showing their differential localization on GC dendrite and in the hilus. Baclofen enhanced the GC population spike to a larger extent in the GABA(B1b)(-/-) mice, demonstrating exclusively disinhibitory roles of the GABA(B(1a,2)) receptors. Conversely, in the GABA(B1a)(-/-) mice baclofen not only enhanced but also inhibited the population spike during GABAA blockade, revealing both disinhibitory and inhibitory effects of GABA(B(1b,2)) receptors.
Conclusions and implications: The GABA(B(1a,2)) and GABA(B(1b,2)) receptor subtypes differentially modulate GC outputs via selective axonal terminal and dendritic locations in the hilar pathways. The GABA(B(1a,2)) receptors exclusively mediate disinhibition, thereby playing a greater role in gating signal transfer for hippocampal spatial and pattern learning.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

Similar articles
-
GABAB-Receptor-mediated currents in interneurons of the dentate-hilus border.J Neurophysiol. 1999 Sep;82(3):1438-50. doi: 10.1152/jn.1999.82.3.1438. J Neurophysiol. 1999. PMID: 10482760
-
Interactive effects of AM251 and baclofen on synaptic plasticity in the rat dentate gyrus.Brain Res. 2016 Nov 15;1651:53-60. doi: 10.1016/j.brainres.2016.09.029. Epub 2016 Sep 20. Brain Res. 2016. PMID: 27663967
-
The GABAB1a isoform mediates heterosynaptic depression at hippocampal mossy fiber synapses.J Neurosci. 2009 Feb 4;29(5):1414-23. doi: 10.1523/JNEUROSCI.3697-08.2009. J Neurosci. 2009. PMID: 19193888 Free PMC article.
-
A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system.Prog Neurobiol. 1995 Jul;46(4):423-62. doi: 10.1016/0301-0082(95)00012-k. Prog Neurobiol. 1995. PMID: 8532848 Review.
-
GABAB receptor complex as a potential target for tumor therapy.J Histochem Cytochem. 2012 Apr;60(4):269-79. doi: 10.1369/0022155412438105. Epub 2012 Jan 20. J Histochem Cytochem. 2012. PMID: 22266766 Free PMC article. Review.
Cited by
-
N-acetyltransferase 10 mediates cognitive dysfunction through the acetylation of GABABR1 mRNA in sepsis-associated encephalopathy.Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2410564121. doi: 10.1073/pnas.2410564121. Epub 2024 Aug 27. Proc Natl Acad Sci U S A. 2024. PMID: 39190359
-
Neuroligin-3 Regulates Excitatory Synaptic Transmission and EPSP-Spike Coupling in the Dentate Gyrus In Vivo.Mol Neurobiol. 2022 Feb;59(2):1098-1111. doi: 10.1007/s12035-021-02663-9. Epub 2021 Nov 29. Mol Neurobiol. 2022. PMID: 34845591 Free PMC article.
-
The emerging role of GABAB receptors as regulators of network dynamics: fast actions from a 'slow' receptor?Curr Opin Neurobiol. 2014 Jun;26:15-21. doi: 10.1016/j.conb.2013.10.002. Epub 2013 Nov 19. Curr Opin Neurobiol. 2014. PMID: 24650499 Free PMC article. Review.
-
Secreted amyloid-β precursor protein functions as a GABABR1a ligand to modulate synaptic transmission.Science. 2019 Jan 11;363(6423):eaao4827. doi: 10.1126/science.aao4827. Science. 2019. PMID: 30630900 Free PMC article.
-
Somatostatin Interneurons Recruit Pre- and Postsynaptic GABAB Receptors in the Adult Mouse Dentate Gyrus.eNeuro. 2024 Aug 19;11(8):ENEURO.0115-24.2024. doi: 10.1523/ENEURO.0115-24.2024. Print 2024 Aug. eNeuro. 2024. PMID: 39084907 Free PMC article.
References
-
- Ault B, Nadler JV. Baclofen selectively inhibits transmission at synapses made by axons of Ca3 pyramidal cells in the hippocampal slice. J Pharmacol Exp Ther. 1982;223:291–297. - PubMed
-
- Ben-Ari Y, Cossart R, Bernard C. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28:108–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

